ABSTRACT
Background
Depression hinders obesity treatment; elucidating mechanisms may enable treatment enhancements.
Objectives
The aim was to investigate whether changes in neural targets in the negative affect circuit following psychotherapy mediate subsequent changes in weight and behaviors.
Methods
Adults (n = 108) with obesity and depression were randomly assigned to usual care or an intervention that delivered problem-solving therapy (PST) for depression over 2 mo. fMRI for brain imaging was performed at baseline and 2 mo. BMI, physical activity, and diet were measured at baseline and 12 mo. Mediation analysis assessed between-group differences in neural target changes using t test and correlations between neural target changes and outcome changes (simple and interaction effect) using ordinary least-squares regression.
Results
Compared with usual care, PST led to reductions in left amygdala activation (−0.75; 95% CI: −1.49, −0.01) and global scores of the negative affect circuit (−0.43; −0.81, −0.06), engaged by threat stimuli. Increases in amygdala activation and global circuit scores at 2 mo correlated with decreases in physical activity outcomes at 12 mo in the usual-care group; these relations were altered by PST. In relation to change in leisure-time physical activity, standardized β-coefficients were −0.67 in usual care and −0.01 in the intervention (between-group difference: 0.66; 0.02, 1.30) for change in left amygdala activation and −2.02 in usual care and −0.11 in the intervention (difference: 1.92; 0.64, 3.20) for change in global circuit scores. In relation to change in total energy expenditure, standardized β-coefficients were −0.65 in usual care and 0.08 in the intervention (difference: 0.73; 0.29, 1.16) for change in left amygdala activation and −1.65 in usual care and 0.08 in the intervention (difference: 1.74; 0.85, 2.63) for change in global circuit scores. Results were null for BMI and diet.
Conclusions
Short-term changes in the negative affect circuit engaged by threat stimuli following PST for depression mediated longer-term changes in physical activity. This trial was registered at www.clinicaltrials.gov as NCT02246413 (https://clinicaltrials.gov/ct2/show/NCT02246413).
Keywords: amygdala, negative affect, functional neuroimaging, obesity, depression, weight loss, diet, physical activity
Introduction
Obesity often manifests with comorbid mood disorders such as depression (1). Unfortunately, adults with obesity and comorbid depression face additional psychological, physiological, and behavioral challenges to weight loss that impair adherence and response to current treatment. Emotion dysregulation may be an important barrier to effective weight loss and lifestyle behavior change. Studies have shown that emotion dysregulation is associated with obesity (2), depression (3), and maladaptive behaviors (e.g., overeating and binge eating) (4–8). Emotion dysregulation may limit the effectiveness of weight-loss interventions by increasing vulnerability to maladaptive behaviors that accompany stress and negative affect. Indeed, stress and negative affect can elicit avoidance or maladaptive coping strategies such as overeating and binge eating (7, 9). Moreover, stress is associated with less engagement in physical activity among individuals already exhibiting low levels of physical activity (10).
One promising approach to treating comorbid obesity and depression is to integrate psychotherapy for depression with behavioral weight-loss treatment. The Research Aimed at Improving Both Mood and Weight (RAINBOW) trial demonstrated that, compared with usual care, an integrated collaborative care intervention led to significantly improved weight loss and depressive symptoms over 12 mo (11). However, the intervention effects were modest and heterogeneous (12). In addition, the intervention did not lead to improved physical activity and diet at 12 mo, compared with usual care, and exploration of behavioral change mechanisms showed mixed findings (13). In alignment with the Science of Behavior Change (SOBC) experimental medicine approach (14), further research is warranted to investigate objective and biologically plausible mechanistic targets for treatment enhancement. The available literature suggests promise in a new line of inquiries to better understand the degree to which engagement of specific neural targets relevant to emotion regulation promotes weight loss, or more specifically, physical activity and improved diet among adults with comorbid obesity and depression.
Previous reviews have synthesized the literature on neural circuit dysfunctions characteristic of emotion dysregulation and of mood disorders such as depression (15, 16). For example, depression has been characterized by heightened amygdala activity to threat-related and mood-congruent sad emotion stimuli along with a loss of amygdala regulation by cortical regions such as the anterior cingulate (17). Reduced functional connectivity between the amygdala and anterior cingulate cortex (ACC) has also been observed in response to threat and sad stimuli in unmedicated patients with major depressive disorder (18). Changes in amygdala activity correlate with response to cognitive-behavioral therapy for depression (19, 20) and antidepressants (21). To our knowledge, however, no study has investigated these neural targets in response to depression treatment for weight loss and behavior change among patients with comorbid obesity and depression using a randomized clinical trial (RCT) design.
This proof-of-mechanism pilot RCT leveraged neuroimaging data from the ENGAGE (Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes) study (22), which was conducted in a RAINBOW subsample. The a priori neural targets included neural regions of the negative affect circuit involved in the regulation of negative emotions (threat and sadness). The objective of this study was to investigate whether early changes in these neural targets following problem-solving therapy (PST) for depression over 2 mo mediated treatment outcomes for weight, physical activity, and diet at 12 mo. This is a necessary step before a larger, adequately powered, hypothesis-driven mechanistic study could be designed.
Methods
The Institutional Review Boards of the University of Illinois at Chicago and Stanford University approved the study. All participants provided written informed consent.
Participants
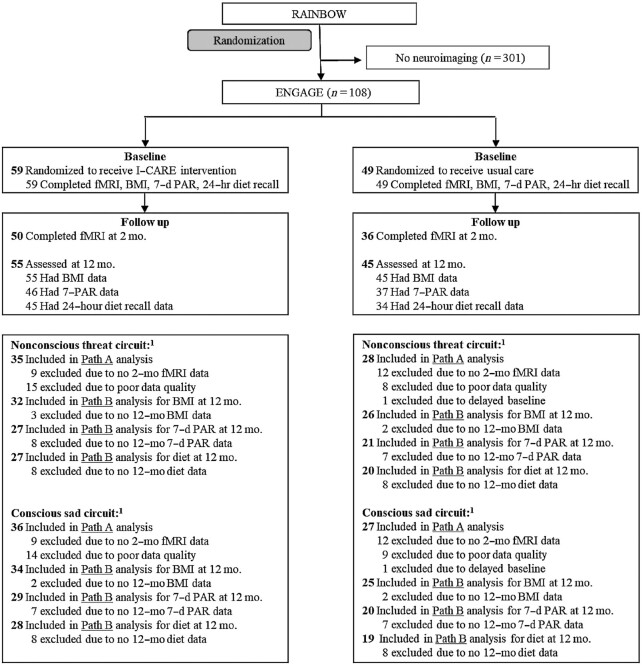
The study sample (n = 108) was a subset of the RAINBOW sample (n = 409) who also consented to the ENGAGE trial (Figure 1). Participants were recruited from family and internal medicine departments in 4 medical centers of Sutter Health's Palo Alto Medical Foundation. Adults of any sex and race/ethnicity were eligible if they had BMI ≥30 (≥27 if Asian) and clinically significant depressive symptoms defined by a 9-item Patient Health Questionnaire (PHQ-9) score ≥10, without serious medical or psychiatric comorbidities or other exclusions. Participants were recruited from 29 November 2015 to 25 October 2016; the date of the final 12-mo follow-up was 30 October 2017. To comply with NIH reporting requirements, participants were asked to self-identify their race and ethnicity from fixed categories.
FIGURE 1.
ENGAGE CONSORT chart. 1Numbers excluded from Path A analyses are relative to participants at baseline; numbers excluded from Path B analyses are relative to participants included in Path A. CONSORT, Consolidated Standards of Reporting Trials; ENGAGE, Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes; I-CARE, Integrated Coaching for Better Mood and Weight; PAR, physical activity recall; RAINBOW, Research Aimed at Improving Both Mood and Weight.
Study procedures, randomization, and blinding
Detailed study procedures were reported in the RAINBOW and ENGAGE trial protocols (22, 23). A preprint of this manuscript was published (24). Funding of this study began after about half of the RAINBOW sample had been enrolled, and subsequent participants were offered the option to enroll in RAINBOW only or in RAINBOW and ENGAGE during the informed consent. RAINBOW participants (including those who also enrolled in ENGAGE) were randomly assigned to receive the Integrated Coaching for Better Mood and Weight (I-CARE) intervention or usual care using a validated online system (25) based on Pocock's covariate-adaptive minimization (26). This method was used to achieve better-than-chance marginal balance across multiple baseline characteristics: clinic, age, sex, race/ethnicity, education, BMI, depression Symptom Checklist 20-items (SCL-20) score, currently taking antidepressant medication, and number of hospitalizations in the past year. After this study began, participants’ choice of enrolling in RAINBOW only or in RAINBOW and ENGAGE was added as a balancing factor. Investigators not involved in delivery of the study intervention, the data and safety monitoring board, outcome assessors, and the data analyst were blinded to participants’ treatment assignment until the end of the study.
Intervention
I-CARE was a 12-mo intervention that combined the Group Lifestyle Balance (GLB) program (27) for weight loss and PST involving the 7-step problem-solving and behavioral activation strategies as first-line, plus antidepressant medications as needed, for depression care management (28, 29). The first 2 mo of intervention focused on depression only with 6 in-person individual PST sessions. This was followed by 3 additional PST sessions for depression and 11 home GLB videos on lifestyle changes for weight loss by 6 mo and 6 maintenance calls by 12 mo. According to the RAINBOW medication protocol, the study supervising psychiatrist followed stepped depression-care strategies and recommended initiating or adjusting antidepressant medications for intervention participants with unremitting symptoms (e.g., PHQ-9 score >50% of baseline by week 10). Therefore, it was unlikely for antidepressant medication initiation or adjustment to have occurred by month 2 of the intervention. [See details in the RAINBOW protocol (23) and Supplemental Table 1.]
Usual care
Participants in both the intervention and usual-care control groups were advised to continue their routine health care. All participants also received information on mental health services and weight-management and other wellness programs routinely available at their clinic. Control participants received a wireless activity tracker with batteries but not any other intervention materials. The activity tracker was used as a self-monitoring tool, not for outcome measurement.
Outcome measures
Outcome measures included BMI as the primary outcome and physical activity and dietary intake as secondary outcomes, all prespecified in the protocol (23) and measured at baseline and 12 mo. Following standardized protocols (30), trained research staff measured height (baseline only) and body weight. BMI was calculated as weight (kg) divided by height squared (m2).
Trained staff interviewed participants to conduct one 7-d Physical Activity Recall (31) and one multiple-pass 24-h dietary recall using the Nutrition Data System for Research (NDSR) (32, 33). The validity of these measures was assessed in diverse populations, including those with obesity or other comorbidities (34, 35). Physical activity was assessed as metabolic equivalent task (MET) minutes per week of leisure-time physical activity of at least moderate intensity based on the sum of the weighted physical activity minutes for moderate (weight: 4 METs), hard (weight: 6 METs), and very hard (weight: 10 METs) activities from the 7-d Physical Activity Recall (36, 37). Also, total energy expenditure provides estimates of total daily energy expenditures in kilocalories per kilogram per day and was derived from MET-minutes/day using the conversion 1 MET = 1 kcal ⸱ kg−1 ⸱ h−1(36, 37). NDSR data provided total caloric intake (kilocalories/day), and as an index of overall diet quality, the DASH (Dietary Approach to Stop Hypertension) score was computed based on 9 nutrient targets (total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium and potassium) (38) provided by the NDSR software.
Potential mediators: neural targets
Participants underwent functional neuroimaging using an established facial emotion task at baseline and 2 mo. The decision to add the 2-mo follow-up time point for neuroimaging was based on current knowledge about the timescale of activity-dependent brain plasticity (39). We were specifically interested in this early timescale within which the intervention could initially modify neural circuit function and for which neural changes might lead to subsequent health outcomes.
Imaging sequences
fMRI was performed at baseline and 2 mo. We implemented previously established functional neuroimaging sequences and parameters as defined in our prespecified protocol (see Supplemental Methods for details) (22).
Viewing of facial emotion task
The negative affect circuit of interest, with an a priori focus on the amygdala, insula, and ACC, was engaged using an established viewing of facial emotion task. Standardized sets of 3D evoked facial expressions of emotion stimuli were presented in pseudorandom order, with 5 repeated blocks of 8 stimuli per block for each emotion relative to neutral blocks (40).
During the conscious viewing condition, each face was presented for 500 ms, with an interstimulus interval of 750 ms. We created a context for participants to continuously view the faces by instructing them that they would be asked post-scan questions about these faces. To elicit the negative affect circuit in response to nonconscious threat stimuli, we presented the same fear and anger stimuli in a backward-masking design to prevent awareness. In this nonconscious condition, face stimuli were presented for 10 ms followed immediately by a neutral face mask stimulus for 150 ms, and with a stimulus onset asynchrony of 1250 ms to match that of the conscious condition (40). Informed by findings for depression we focused on the nonconscious viewing condition for threat and the conscious viewing condition for sad (15, 16).
Pre-processing
Pre-processing of functional data included realignment and unwarping, normalization to a standardized template, and 8-mm Full Width Half Maximum Gaussian smoothing. Quality-control diagnostics included removing scans with incidental findings, scanner artefacts, and signal dropout. Participants’ data were included if no more than 25% (38/151) of time points were censored for frame-wise displacement or variance spikes. This resulted in a total of n = 89 and n = 74 for the baseline and 2-mo imaging sessions, respectively.
Defining and quantifying primary and secondary neural target regions of interests
A priori–defined primary target regions of interest (ROIs) were the subgenual ACC (sgACC) and amygdala (bilaterally) for threat and the pregenual ACC (pgACC), amygdala (bilaterally), and anterior insula (bilaterally) for sad. Patient-level activation of the ROIs for the contrast of each emotion (threat or sadness) minus neutral was derived in a manner consistent with the methods used for a healthy reference sample (21, 40–42).
Secondary neural targets focused on functional connectivity with the subcortical regions of primary interest. Region-to-region connectivity for nonconscious threat was focused on connections between the amygdala and the sgACC. For sad, we focused on connections between the amygdala and the pgACC as well as the anterior insula and pgACC. Functional connectivity was computed using a psychophysiological interaction (PPI) procedure (see Supplemental Methods for details). Similarly, PPI analyses were used to quantify functional connectivity between ROIs (43). Quantification of connectivity followed the previously established systematic procedure (41).
Each of the activation and connectivity values was expressed in SD units relative to the healthy reference sample (41). Through this procedure, activation values were interpretable relative to a healthy reference mean of zero. Then the values were winsorized using ±3 SDs.
Also, of secondary interest was to provide an overall neural measure of negative affect circuit function, where we computed a global circuit score that combined activation and connectivity of constituent primary regions and the cortical regions to which they connect. This global score was an average of the standardized values for each constituent measure (see Supplemental Methods for details) (41, 44). Higher global circuit scores indicate greater dysfunction according to our theoretical framework (15, 16).
Statistical analysis
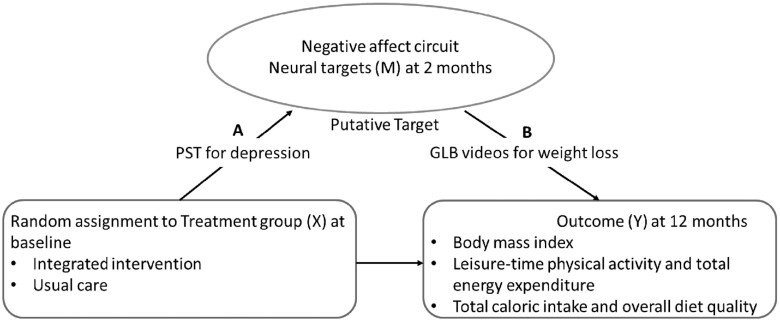
Longitudinal mediation analysis was conducted to assess whether change in a potential mediator from baseline to 2 mo mediated change in an outcome from baseline to 12 mo. This analytical approach was also used in a recent publication (45) based on data from the same trial to assess whether changes in the same neural measures mediated changes in depression and problem-solving outcomes. According to Kraemer et al. (46), we defined that mediation exists if a potential mediator meets the following 2 conditions: First, there is a significant effect of the intervention compared with control (X) on the potential mediator (M, X→M Path A). Second, the potential mediator is significantly associated with the outcome either as a simple effect in the usual-care group or an interaction effect with the intervention relative to the usual-care group (M→Y Path B) (Figure 2).
FIGURE 2.
(A, B) Conceptual framework for mediation analysis. Primary neural targets: sgACC and amygdala (bilaterally) for nonconscious threat stimuli, and the pgACC, amygdala (bilaterally), and anterior insula (bilaterally) for conscious sad stimuli. Secondary neural targets: connectivity between sgACC and bilateral amygdala and global circuit score for nonconscious threat stimuli, and connectivity between pgACC and bilateral amygdala, connectivity between pgACC and bilateral anterior insula, and global circuit score for conscious sad stimuli. GLB, Group Lifestyle Balance; pgACC, pregenual anterior cingulate cortex; PST, problem-solving therapy; sgACC, subgenual anterior cingulate cortex.
Path A was assessed using the t test to obtain mean differences and 95% CIs. For Path B, the ordinary least-squares regression (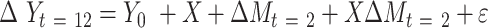 ) was used to test the association of change in a potential mediator at 2 mo (
) was used to test the association of change in a potential mediator at 2 mo (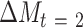 ) with change in an outcome at 12 mo (
) with change in an outcome at 12 mo (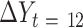 ) within the usual-care group (simple effect, X with usual care = 0, intervention = 1) and the difference in the size of the treatment effect between the intervention and usual-care groups at different levels of the mediator (interaction effect,
) within the usual-care group (simple effect, X with usual care = 0, intervention = 1) and the difference in the size of the treatment effect between the intervention and usual-care groups at different levels of the mediator (interaction effect, 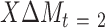 ), adjusting for the baseline value of the outcome measure (
), adjusting for the baseline value of the outcome measure (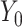 ). To do this, each outcome was standardized using the baseline SD of the RAINBOW sample for uniform comparison of effect sizes across measures with different units. Complementarily, associations of changes in neural targets at 2 mo with changes in outcomes in the original scales are presented in Figure 3. Completer analyses were conducted using data from participants who completed fMRI at baseline and 2 mo (Path A analyses) as well as the outcome measures at 12 mo (Path B analyses) (see Figure 1 for numbers of subjects included in all the analyses). Participants were analyzed based on the group to which they were randomly assigned.
). To do this, each outcome was standardized using the baseline SD of the RAINBOW sample for uniform comparison of effect sizes across measures with different units. Complementarily, associations of changes in neural targets at 2 mo with changes in outcomes in the original scales are presented in Figure 3. Completer analyses were conducted using data from participants who completed fMRI at baseline and 2 mo (Path A analyses) as well as the outcome measures at 12 mo (Path B analyses) (see Figure 1 for numbers of subjects included in all the analyses). Participants were analyzed based on the group to which they were randomly assigned.
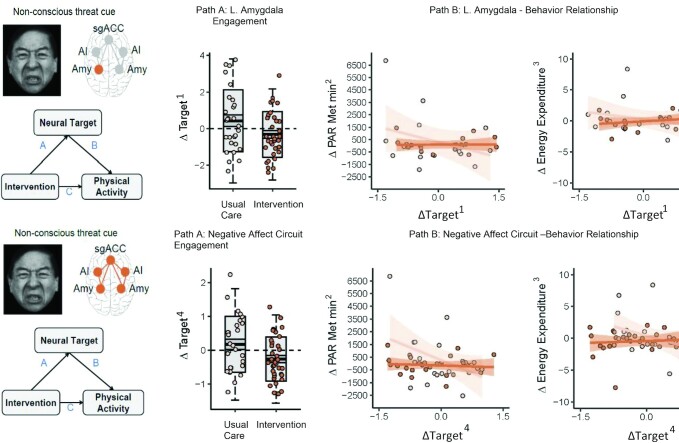
FIGURE 3.
Problem-solving therapy–induced negative affect circuit engagement at 2 mo mediates subsequent physical activity improvements at 12 mo. (Path A) Early change in neural target engagement for intervention versus usual care at 2 mo, assessed using the t test. (Path B) Association of early change in neural target engagement at 2 mo with subsequent change in leisure-time physical activity and total energy expenditure at 12 mo, tested using ordinary least-squares regression. All changes are relative to baseline. Path A and Path B results are represented by dark-orange circles and lines for the intervention group and light-orange circles and lines for the usual-care group. For the box plots, the central thick black bar represents the mean, gray-shaded boxes represent SE (dark gray) and SD (lighter gray) of the mean, and the whiskers represent 2 SDs of the mean. The impacts on the intervention on physical activity are reported in Rosas et al. (13) for the full RAINBOW trial. 1BOLD activation vs. neutral cue, z-scored. 2The interview-administered Stanford 7-d Physical Activity Recall provided MET minutes/week for leisure moderate activity. Leisure-time physical activity = non–work-related moderate activity minutes/week × 4 METs + hard activity minuntes/week × 6 METs + very hard activity minutes/week × 10 METs. One MET is defined as the energy expenditure for sitting quietly. 3The Stanford 7-d Physical Activity Recall data also provided estimates of total daily energy expenditures. Total energy expenditure = sleep hours × 1 MET + light activity hours × 1.5 METs + moderate activity hours × 4 METs + hard activity hours × 6 METs + very hard activity hours × 10 METs. 4Overall score from BOLD derived activation and connectivity within the negative affective circuit. L, left; MET, metabolic equivalent task; PAR, physical activity recall .
To aid transparent interpretation, we present P values unadjusted and adjusted (Padj) using the false discovery rate (FDR) procedure (47) to control the family error rates across tests of similar hypotheses. Specifically, for each path in a given mediation model, we grouped neural targets by category (primary targets engaged by threat and sad stimuli separately, and secondary targets) and applied the FDR procedure across the resulting family of related tests for that path (see Supplemental Table 2 for families of tests used to calculate FDR-adjusted P values). Due to the pilot nature of this proof-of-mechanism study, we focused on reporting standardized mean estimates with 95% CIs as recommended in the American Statistical Association Statement and recent position paper (48, 49) and discussing effects of medium or larger size, especially where there were consistent findings across related measures.
All analyses were conducted using SAS, version 9.4 (SAS Institute, Inc.). Figures were generated using the ggplot2 package in R (version 3.5.0; R Foundation for Statistical Computing).
Sample size calculation
This mechanistic pilot trial was, by design, not fully powered to test focused hypotheses about the existence of particular mediators. Rather, it was designed to generate strong hypotheses for future experimental studies that would formally test a priori hypotheses about specific mediators in relation to the cause and effect of a treatment on an outcome. Accordingly, the sample size was determined by operational feasibility (e.g., the start of this study in relation to the time course in the then-ongoing parent RAINBOW trial and available funding) and the design choice to focus on medium or larger effects given the likely limited clinical relevance of small effects. When considering all combinations of treatment-to-mediator (Path A) and mediator-to-outcome (Path B) effects in a suitable effect size metric, S = 0.14 is small (akin to Cohen's d = 0.20), H = 0.26 is halfway between small and medium (d = 0.35), M = 0.39 is medium (d = 0.50), and L = 0.59 is large (d = 0.80) (50). Considering the medium to large effect size combinations of HH, HM, MH, and MMfor the joint mediation Path A and Path B, a sample size of at least 100 participants for the ENGAGE subsample was selected to detect medium effect sizes (Cohen's d: 0.3–0.5) in predicting treatment outcomes assuming 80% completion rates.
Results
Sample characteristics
Baseline characteristics of the 108 participants are shown in Table 1. Similar to the overall RAINBOW sample (11), ENGAGE participants had moderately severe obesity, a mean BMI of 35.5 (SD: 5.1), and moderate depression (mean SCL-20 score: 1.5; SD: 0.5).
TABLE 1.
Baseline characteristics by treatment group1
| Intervention (n = 59) | Usual care (n = 49) | |
|---|---|---|
| Clinics in the Bay Area,2n (%) | ||
| Los Altos | 2 (3) | 1 (2) |
| Sunnyvale | 3 (5) | 3 (6) |
| Palo Alto | 36 (61) | 29 (59) |
| Mountain View | 18 (31) | 16 (33) |
| Age,2 mean (SD), y | 52.4 (11.6) | 51.6 (12.0) |
| Sex,2n (%) | ||
| Female | 42 (71) | 31 (63) |
| Male | 17 (29) | 18 (37) |
| Race/ethnicity,2n (%) | ||
| Non-Hispanic White | 46 (78) | 35 (71) |
| Black | 1 (2) | 0 (0) |
| Asian/Pacific Islander | 5 (8) | 3 (6) |
| Hispanic | 4 (7) | 7 (14) |
| Other3 | 3 (5) | 4 (8) |
| Education,2n (%) | ||
| High school graduate or GED | 2 (3) | 4 (8) |
| Some college | 10 (17) | 14 (29) |
| Undergraduate degree | 28 (47) | 15 (31) |
| Graduate level work or degree | 19 (32) | 16 (33) |
| BMI,2 mean (SD), kg/m2 | ||
| Both sexes | 34.9 (5.2) | 36.3 (4.9) |
| Women | 35.2 (5.5) | 37.6 (5.2) |
| Men | 34.2 (4.2) | 34.0 (3.5) |
| Weight, mean (SD), kg | ||
| Both sexes | 98.8 (17.1) | 104.2 (13.2) |
| Women | 94.7 (16.5) | 100.8 (13.4) |
| Men | 108.9 (14.5) | 109.9 (11.0) |
| Height, mean (SD), cm | ||
| Both sexes | 168.1 (10.1) | 169.8 (10.6) |
| Women | 163.9 (8.4) | 163.8 (7.2) |
| Men | 178.4 (5.7) | 180.0 (7.0) |
| PHQ-9 score, mean (SD)4 | 14.0 (3.1) | 13.4 (2.9) |
| SCL-20 score, mean (SD)2,5 | 1.5 (0.6) | 1.6 (0.5) |
| GAD-7 score, mean (SD)6 | 7.8 (4.3) | 8.02 (5.0) |
| Taking antidepressant medications,2,7n (%) | 24 (41) | 19 (39) |
| Hospitalized during the last year,2,7 n (%) | 7 (12) | 4 (8) |
| Depression diagnosis or treatment, n (%) | 39 (66) | 33 (67) |
| Employment status, n (%) | ||
| Full-time | 34 (58) | 28 (57) |
| Part-time | 10 (17) | 5 (10) |
| Unemployed | 15 (25) | 16 (33) |
| Income, n/total n (%) | ||
| <$75,000 | 8/53 (15) | 15/46 (33) |
| $75,000–$150,000 | 21/53 (40) | 13/46 (28) |
| ≥$150,000 | 24/53 (45) | 18/46 (39) |
| Insurance, n (%) | ||
| Preferred provider organization | 42 (71) | 27 (55) |
| Health management organization | 12 (20) | 18 (37) |
| Medicare fee for service | 5 (8) | 2 (4) |
| Other insurance | 0 (0) | 2 (4) |
| Marital status, n (%) | ||
| Married or living with a partner | 35 (59) | 30 (61) |
| Single, separated, divorced, or widowed | 24 (41) | 19 (39) |
| Household size, n (%) | ||
| <2 | 14 (24) | 7 (14) |
| 2 | 23 (39) | 20 (41) |
| ≥ 3 | 22 (37) | 22 (45) |
GAD-7, 7-Item Generalized Anxiety Disorder; GED, general equivalency diploma; PHQ-9, 9-item Patient Health Questionnaire; SCL-20, Depression Symptom Checklist 20-items.
Randomization balancing factors.
Includes 1 participant reporting mixed race and 2 reporting other race (unspecified) in the intervention group; 4 participants reporting mixed race in the usual-care control group.
PHQ-9, total scores of the 9 items with a range from 0 (no symptoms) to 27 (most severe symptoms). PHQ-9 cutoff points of 10, 15, and 20 correspond to moderate, moderately severe, and severe depression, respectively.
SCL-20, total average scores of the 20 items with a range from 0 (not at all) to 4 (extremely). SCL-20 cutoff points of 1.5 and 2.0 correspond to moderate and severe depression, respectively.
GAD-7, total scores of the 7 items with a range from 0 (no symptoms) to 21 (most severe symptoms). GAD-7 cutoff points of 5, 10, and 15 correspond to mild, moderate, and severe anxiety, respectively.
Based on patient self-report at screening.
Neural targets engaged by threat-related stimuli
Intervention effect on neural target changes (Path A)
Among the primary neural targets engaged by threat stimuli, mean changes in amygdala activity from baseline to 2 mo were −0.75 (95% CI: −1.49, −0.01; P = 0.049, Padj = 0.10) for the left amygdala and −0.63 (95% CI: −1.30, 0.05; P = 0.07, Padj = 0.10) for the right amygdala in the intervention compared with the usual-care group (Table 2 and Figure 3). In addition, the mean change in global circuit scores from baseline to 2 mo was −0.43 (95% CI: −0.81, −0.06; P = 0.02, Padj = 0.18) in the intervention compared with the usual-care group. While these results suggest medium effects, the Padj values are all >0.05. Results for changes in sgACC activity and connectivity between the sgACC and the bilateral amygdala from baseline to 2 mo showed no evidence for differences between the intervention and usual-care groups.
TABLE 2.
Treatment effects on the potential mediators (Path A)1
| Unadjusted mean (SD) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Intervention3 | Usual care | Intervention difference4 | ||||||
| Circuit and neural target | Hemi2 | Baseline | Δ 2 mo | Baseline | Δ 2 mo | Mean [95% CI] | P | FDR Padj |
| Threat (nonconscious)5 | ||||||||
| Amygdala | L | 0.30 (0.96) | −0.32 (1.25) | 0.09 (1.00) | 0.43 (1.69) | −0.75 [−1.49, −0.01]* | 0.049* | 0.10* |
| R | 0.26 (0.75) | −0.07 (1.38) | 0.01 (0.84) | 0.56 (1.25) | −0.63 [−1.30, 0.05]* | 0.07* | 0.10* | |
| sgACC | M | 0.24 (1.08) | 0.22 (1.18) | −0.11 (0.99) | 0.46 (1.30) | −0.24 [−0.87, 0.39] | 0.45 | 0.45 |
| sgACC to amygdala | M–L | −0.37 (0.80) | 0.36 (1.25) | 0.20 (0.94) | −0.22 (1.21) | 0.58 [−0.05, 1.20] | 0.07 | 0.27 |
| M–R | −0.23 (0.83) | 0.37 (1.17) | 0.25 (0.90) | −0.13 (1.27) | 0.49 [−0.12, 1.11] | 0.12 | 0.31 | |
| Circuit6 | — | 0.18 (0.44) | −0.26 (0.65) | −0.05 (0.56) | 0.18 (0.82) | −0.43 [−0.81, −0.06]* | 0.02* | 0.18* |
| Sad (conscious)7 | ||||||||
| Amygdala | L | −0.20 (0.91) | 0.05 (1.22) | −0.29 (0.90) | 0.27 (1.36) | −0.22 [−0.87, 0.43] | 0.50 | 0.78 |
| R | −0.03 (0.70) | 0.01 (0.99) | −0.10 (0.83) | 0.30 (1.17) | −0.29 [−0.84, 0.25] | 0.29 | 0.78 | |
| Anterior insula | L | 0.02 (0.67) | −0.18 (0.98) | 0.04 (0.63) | −0.09 (1.27) | −0.09 [−0.66, 0.48] | 0.76 | 0.78 |
| R | 0.00 (0.73) | 0.02 (0.95) | −0.01 (0.74) | −0.06 (1.34) | 0.08 [−0.50, 0.66] | 0.78 | 0.78 | |
| pgACC | M | −0.11 (0.85) | −0.36 (1.13) | −0.20 (0.94) | −0.10 (1.29) | −0.26 [−0.87, 0.36] | 0.41 | 0.78 |
| pgACC to amygdala | M–L | −0.12 (0.96) | 0.38 (1.49) | −0.23 (0.86) | 0.23 (1.33) | 0.15 [−0.58, 0.87] | 0.69 | 0.79 |
| M–R | 0.05 (0.91) | 0.15 (1.40) | −0.07 (0.81) | −0.06 (1.21) | 0.21 [−0.47, 0.88] | 0.54 | 0.72 | |
| pgACC to anterior insula | M–L | −0.10 (0.68) | −0.01 (1.16) | −0.35 (0.82) | 0.21 (1.08) | −0.22 [−0.80, 0.35] | 0.44 | 0.72 |
| M–R | −0.20 (0.78) | 0.02 (1.29) | −0.53 (0.81) | 0.23 (0.97) | −0.22 [−0.81, 0.38] | 0.47 | 0.72 | |
| Circuit6 | — | −0.01 (0.31) | 0.01 (0.53) | 0.00 (0.30) | 0.00 (0.53) | 0.01 [−0.27, 0.27] | 0.97 | 0.97 |
Path A was assessed using the t test to obtain mean differences and 95% CIs. *Results reported in the manuscript text. FDR, false discovery rate; Hemi, hemisphere; I-CARE, Integrated Coaching for Better Mood and Weight; L, left; M, medial; P, P value at an uncorrected threshold of 0.05 prior to adjustment for FDR; Padj., P value adjusted for FDR within neural target family (see Supplemental Table 2); pgACC, pregenual anterior cingulate cortex; R, right; sgACC, subgenual anterior cingulate cortex.
Single letter indicates task activation; paired letters indicate task-related connectivity.
Represents the initial 2-mo intervention phase of the I-CARE program that implemented a 7-step problem-solving process as its core component.
Due to the nature of this mechanistic pilot study, we focused on standardized mean estimates with 95% CIs and presented P values unadjusted and adjusted (Padj) using the FDR procedure to control the family error rates across tests of similar hypotheses.
Analyses on threat circuit were conducted with ntotal = 88 participants (nintervention = 47 and nusual care = 41) at baseline, and with ntotal = 63 participants (nintervention = 35 and nusual care = 28) with baseline and 2-mo follow-up.
Global circuit dysfunction score, composite of primary and secondary neural targets (41).
Analyses on sad circuit were conducted with ntotal = 89 participants (nintervention = 50 and nusual care = 39) at baseline, and with ntotal = 63 participants (nintervention = 36 and nusual care = 27) with baseline and 2-month follow-up.
Association of neural target changes with outcomes (Path B)
Increases in amygdala activity for threat stimuli from baseline to 2 mo were associated with decreases in leisure-time physical activity and total energy expenditure from baseline to 12 mo in the usual-care group, but this inverse relation was tempered or reversed in the intervention group (Table 3 and Figure 3). For the left amygdala, the usual-care effect was −0.67 (95% CI: −1.14, −0.21; P = 0.01, Padj = 0.03) and the interaction effect was 0.66 (95% CI: 0.02, 1.30; P = 0.04, Padj = 0.08) for leisure-time physical activity, and the usual-care effect was −0.65 (95% CI: −0.97, −0.33; P < 0.001, Padj = 0.001) and the interaction effect was 0.73 (95% CI: 0.29, 1.16; P = 0.002, Padj = 0.005) for total energy expenditure. For the right amygdala, the usual-care effect was −0.56 (95% CI: −1.04, −0.07; P = 0.03, Padj = 0.05) and the interaction effect was 0.61 (95% CI: 0.03, 1.18; P = 0.04, Padj = 0.06) for total energy expenditure. Similarly, an inverse relation was observed between change in global circuit scores for threat stimuli from baseline to 2 mo and changes in leisure-time physical activity and total energy expenditure from baseline to 12 mo among the usual-care group, which was altered in the intervention group. The usual-care effect was −2.02 (95% CI: −3.05, −1.00; P < 0.001, Padj = 0.005) and the interaction effect was 1.92 (95% CI: 0.64, 3.20; P = 0.004, Padj = 0.03) for leisure-time physical activity, and the usual-care effect was −1.65 (95% CI: −2.36, −0.95; P < 0.001, Padj < 0.001) and the interaction effect was 1.74 (95% CI: 0.85, 2.63; P < 0.001, Padj = 0.002) for total energy expenditure (Table 3 and Figure 3). While these results suggest medium or larger effect sizes, for some, Padj values are >0.05.
TABLE 3.
Associations of changes in potential mediators at 2 mo with changes in outcomes at 12 mo for the nonconscious threat circuit (Path B)1
| Intervention3 | Usual care | Between-group difference | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Component name | Hemi2 | Mean | [95% CI] | P | FDR Padj. | Mean | [95% CI] | P | FDR Padj. | Mean | [95% CI] | P | FDR Padj. |
| BMI | |||||||||||||
| Amygdala | L | −0.03 | [−0.12, 0.06] | 0.56 | 0.56 | 0.04 | [−0.05, 0.12] | 0.39 | 0.77 | −0.06 | [−0.18, 0.06] | 0.31 | 0.77 |
| R | −0.05 | [−0.14, 0.03] | 0.21 | 0.56 | 0.01 | [−0.10, 0.12] | 0.81 | 0.83 | −0.07 | [−0.20, 0.07] | 0.33 | 0.77 | |
| sgACC | M | −0.03 | [−0.13, 0.07] | 0.54 | 0.56 | −0.02 | [−0.11, 0.09] | 0.76 | 0.83 | −0.02 | [−0.15, 0.12] | 0.83 | 0.83 |
| sgACC to amygdala | M–L | −0.10 | [−0.2, −0.01] | 0.03 | 0.25 | 0.00 | [−0.10, 0.11] | 0.97 | 0.97 | −0.10 | [−0.24, 0.03] | 0.13 | 0.55 |
| M–R | −0.09 | [−0.19, 0.00] | 0.06 | 0.25 | 0.01 | [−0.09, 0.11] | 0.79 | 0.97 | −0.10 | [−0.24, 0.03] | 0.14 | 0.55 | |
| Circuit4 | — | 0.08 | [−0.10, 0.26] | 0.37 | 0.92 | 0.04 | [−0.13, 0.21] | 0.65 | 0.97 | 0.04 | [−0.20, 0.28] | 0.72 | 0.97 |
| Leisure-time physical activity(MET-min/wk) | |||||||||||||
| Amygdala | L | −0.01 | [−0.44, 0.42] | 0.95 | 0.95 | −0.67* | [−1.14, −0.21]* | 0.01* | 0.03* | 0.66* | [0.02, 1.30]* | 0.04* | 0.08* |
| R | −0.04 | [−0.48, 0.39] | 0.84 | 0.95 | −0.50 | [−1.18, 0.18] | 0.14 | 0.17 | 0.45 | [−0.35, 1.26] | 0.26 | 0.26 | |
| sgACC | M | −0.11 | [−0.63, 0.42] | 0.69 | 0.95 | 0.62 | [0.10, 1.13] | 0.02 | 0.06 | −0.72 | [−1.46, 0.01] | 0.05 | 0.08 |
| sgACC to amygdala | M–L | 0.10 | [−0.40, 0.59] | 0.70 | 0.78 | 0.35 | [−0.36, 1.05] | 0.33 | 0.63 | −0.25 | [−1.11, 0.61] | 0.56 | 0.82 |
| M–R | 0.10 | [−0.43, 0.63] | 0.71 | 0.78 | 0.29 | [−0.34, 0.91] | 0.36 | 0.63 | −0.19 | [−1.01, 0.62] | 0.64 | 0.85 | |
| Circuit4 | — | −0.11 | [−0.87, 0.66] | 0.78 | 0.78 | −2.02* | [−3.05, −1.00]* | <0.001* | 0.005* | 1.92* | [0.64, 3.20]* | 0.004* | 0.03* |
| Energy expenditure(kcal/kg/d) | |||||||||||||
| Amygdala | L | 0.08 | [−0.22, 0.37] | 0.60 | 0.86 | −0.65* | [−0.97, −0.33]* | <0.001* | 0.001* | 0.73* | [0.29, 1.16]* | 0.002* | 0.005* |
| R | 0.05 | [−0.26, 0.36] | 0.74 | 0.86 | −0.56* | [−1.04, −0.07]* | 0.03* | 0.05* | 0.61* | [0.03, 1.18]* | 0.04* | 0.06* | |
| sgACC | M | −0.04 | [−0.44, 0.37] | 0.86 | 0.86 | 0.32 | [−0.08, 0.72] | 0.12 | 0.14 | −0.35 | [−0.92, 0.22] | 0.22 | 0.22 |
| sgACC to amygdala | M–L | 0.02 | [−0.35, 0.39] | 0.91 | 0.91 | 0.29 | [−0.20, 0.77] | 0.24 | 0.72 | −0.27 | [−0.87, 0.34] | 0.38 | 0.72 |
| M–R | 0.02 | [−0.37, 0.41] | 0.91 | 0.91 | 0.27 | [−0.19, 0.73] | 0.24 | 0.72 | −0.25 | [−0.85, 0.35] | 0.41 | 0.72 | |
| Circuit4 | — | 0.08 | [−0.46, 0.62] | 0.76 | 0.91 | −1.65* | [−2.36, −0.95]* | <0.001* | <0.001* | 1.74* | [0.85, 2.63]* | <0.001* | 0.002* |
| Calorie intake (kcal/d) | |||||||||||||
| Amygdala | L | 0.04 | [−0.15, 0.23] | 0.68 | 0.71 | 0.04 | [−0.17, 0.25] | 0.70 | 1.00 | −0.00 | [−0.28, 0.28] | 1.00 | 1.00 |
| R | −0.03 | [−0.22, 0.15] | 0.71 | 0.71 | 0.00 | [−0.29, 0.30] | 0.99 | 1.00 | −0.04 | [−0.38, 0.31] | 0.84 | 1.00 | |
| sgACC | M | 0.10 | [−0.12, 0.31] | 0.38 | 0.71 | −0.16 | [−0.38, 0.06] | 0.15 | 0.45 | 0.26 | [−0.05, 0.57] | 0.10 | 0.45 |
| sgACC to amygdala | M–L | −0.01 | [−0.21, 0.20] | 0.94 | 0.96 | −0.02 | [−0.30, 0.27] | 0.91 | 0.96 | 0.01 | [−0.34, 0.35] | 0.96 | 0.96 |
| M–R | −0.01 | [−0.23, 0.20] | 0.92 | 0.96 | −0.06 | [−0.32, 0.20] | 0.63 | 0.78 | 0.05 | [−0.29, 0.39] | 0.76 | 0.87 | |
| Circuit4 | — | −0.03 | [−0.40, 0.33] | 0.86 | 0.96 | 0.25 | [−0.24, 0.73] | 0.31 | 0.60 | −0.28 | [−0.88, 0.32] | 0.35 | 0.60 |
| DASH score5 | |||||||||||||
| Amygdala | L | 0.01 | [−0.31, 0.33] | 0.93 | 0.93 | −0.16 | [−0.52, 0.19] | 0.36 | 0.44 | 0.17 | [−0.30, 0.65] | 0.47 | 0.47 |
| R | −0.06 | [−0.36, 0.25] | 0.71 | 0.93 | −0.35 | [−0.85, 0.14] | 0.16 | 0.44 | 0.30 | [−0.28, 0.88] | 0.31 | 0.44 | |
| sgACC | M | 0.04 | [−0.34, 0.42] | 0.83 | 0.93 | −0.22 | [−0.61, 0.16] | 0.25 | 0.44 | 0.27 | [−0.28, 0.81] | 0.33 | 0.44 |
| sgACC to amygdala | M–L | 0.10 | [−0.25, 0.44] | 0.57 | 0.78 | 0.34 | [−0.14, 0.82] | 0.16 | 0.51 | −0.24 | [−0.83, 0.35] | 0.41 | 0.74 |
| M–R | 0.24 | [−0.12, 0.61] | 0.19 | 0.78 | 0.06 | [−0.38, 0.50] | 0.79 | 0.85 | 0.19 | [−0.40, 0.77] | 0.52 | 0.83 | |
| Circuit4 | — | −0.26 | [−0.88, 0.35] | 0.39 | 0.78 | −0.41 | [−1.23, 0.41] | 0.32 | 0.64 | 0.15 | [−0.88, 1.17] | 0.77 | 0.85 |
Analyses on threat circuit were conducted with ntotal = 88 participants (nintervention = 47 and nusual care = 41) at baseline, and with ntotal = 63 participants (nintervention = 35 and nusual care = 28) with baseline and 2-mo follow-up. Path B was tested using ordinary least-squares regression to assess the association of changes in a potential mediator at 2 mo with changes in an outcome in the usual-care group (the usual-care effect) and by interaction with the intervention relative to the usual-care group (the interaction effect) at 12 mo, adjusting for the baseline value of the outcome measure. Each outcome was standardized using the baseline standard deviation of the RAINBOW sample for uniform comparison of effect sizes across measures with different units. *Results were reported in the manuscript text. DASH, Dietary Approach to Stop Hypertension; FDR, false discovery rate; Hemi, hemisphere; I-CARE, Integrated Coaching for Better Mood and Weight; L, left; M, medial; MET, metabolic equivalent task; P, P value at an uncorrected threshold of 0.05 prior to adjustment for FDR; Padj, P value adjusted for FDR within neural target family (see Supplemental Table 2); R, right; RAINBOW, Research Aimed at Improving Both Mood and Weight; sgACC, subgenual anterior cingulate cortex.
Single letter indicates task activation; paired letters indicate task-related connectivity.
Represents the initial 2-mo intervention phase of the I-CARE program that implemented a 7-step problem-solving process as its core component.
Global circuit dysfunction score, composite of primary and secondary neural circuit targets.
DASH scores were calculated based on combining nine nutrient targets (i.e., total fat, saturated fat, protein, cholesterol, fiber, magnesium, calcium, sodium, and potassium). The intermediate target of each nutrient was halfway between the DASH target and population mean (based on the NHANES 2007–2008, latest data available at the inception of the study). For a nutrient, participants reaching the DASH target were assigned 1 point, those reaching the intermediate target were assigned a half-point, and those not meeting the intermediate target were given 0 points. The DASH score was the sum of points for all 9 nutrients.
Results for associations between the remaining neural target changes engaged by threat stimuli and changes in leisure-time physical activity and total energy expenditure and between changes in any neural targets engaged by threat stimuli and changes in BMI, total caloric intake, and DASH scores were null (Table 3).
Neutral targets engaged by sad stimuli
Intervention effect on neural target changes (Path A)
There was no evidence that changes in any of the primary and secondary neural targets engaged by sad stimuli from baseline to 2 mo differed between the intervention and the usual-care groups.
Association of neural target changes with outcomes (Path B)
Decreased connectivity between the pgACC and the left anterior insular engaged by sad stimuli from baseline to 2 mo was associated with increased caloric intake from baseline to 12 mo in the usual-care group (−0.40; 95% CI: −0.71, −0.08; P = 0.02, Padj = 0.12), but this inverse relation was reversed in the intervention group (0.47; 95% CI: 0.10, 0.84; P = 0.01, Padj = 0.12). Decreased connectivity between the pgACC and the right anterior insular engaged by sad stimuli from baseline to 2 mo was associated with decreased overall dietary quality based on change in DASH scores from baseline to 12 mo in the usual-care group (0.62; 95% CI: 0.03, 1.20; P = 0.04, Padj = 0.31), but this relation was reversed in the intervention group (−0.72; 95% CI: −1.38, −0.07; P = 0.03, Padj = 0.31) (Supplemental Table 3). The observed effects were of medium size, but the Padj values are all >0.05.
Results for associations between the remaining neural target changes engaged by sad stimuli and changes in caloric intake and overall dietary quality and between changes in any neural targets engaged by sad stimuli and changes in BMI, leisure-time physical activity, and total energy expenditure were null (Supplemental Table 3).
Discussion
This proof-of-mechanism pilot study found that neural target changes in the negative affect circuit in response to early PST for depression could mediate or associate with subsequent changes in physical activity and diet. First, reductions in left amygdala activity and global circuit scores for threat stimuli following 2 mo of PST for depression mediated subsequent improvements in leisure-time physical activity and total energy expenditure at 12 mo. These results were demonstrated for both Paths A and B, thereby meeting the study definition of mediation. Second, increases in right amygdala activity for threat stimuli at 2 mo associated with decreases in total energy expenditure at 12 mo in the usual-care group, but this inverse relation was reversed in the intervention group. Third, decreased functional connectivity between pgACC and anterior insula for sad stimuli at 2 mo was associated with increased caloric intake and decreased overall dietary quality at 12 mo in the usual-care group, and PST for depression reversed these relations. However, changes in right amygdala activity for threat stimuli and connectivity between pgACC and anterior insula for sad stimuli from baseline to 2 mo did not differ significantly between the intervention and the usual-care groups. Cumulatively, these findings suggest that improvements in weight-related health behaviors at the end of the integrated collaborative care intervention for comorbid obesity and depression over 12 mo may depend on early changes in specific neural regions of the negative affect circuit. The results for BMI change at 12 mo did not suggest a meaningful relation with any of the neural targets at 2 mo.
This study showed that early PST for depression during the integrated collaborative care intervention reduced amygdala activity for threat stimuli among intervention compared with usual-care participants by 2 mo. Activity in the amygdala is critical for affective responses to negative stimuli and is altered by depression (3). As such, attenuated threat reactivity in the bilateral amygdala observed in the intervention group relative to usual care at 2 mo may represent the neural signature of a successful response to PST, which, in turn, mediated subsequent improvements in physical activity and energy expenditure at 12 mo. In line with the SOBC experimental medicine approach, these findings offer insights into early engagement and malleability of the posited neural targets with treatment that demonstrate temporal precedence and meaningful relations to downstream outcomes. This alone does not establish causality. Nonetheless, the results can inform the design of an adequately powered, hypothesis-driven mechanistic study in order to confirm the causal role of the identified neural mediators in explaining the treatment effect for the outcomes of interest.
To our knowledge, this is the first study to identify amygdala reactivity to threat stimuli as a mediator for changes in physical activity and energy expenditure. Much of the literature to date has focused on identifying the ability of behavior [e.g., acute exercise (51)] and lifestyle interventions (e.g., Tai Chi, exercise training, weight-loss program) to engage neural circuits of interest (52–55). These prior studies suggest that certain interventions can selectively activate or inhibit neural targets (e.g., default mode, attention/limbic, and executive control networks), but these observations have not been connected to changes in health outcomes. Some data suggest that baseline brain network modularity (i.e., the degree to which a group of neural components are partitioned into subnetworks) (56) may predict individuals’ changes in executive/working memory function following cognitive (57) or exercise-training (58, 59) interventions. Few data to date, however, have connected changes in neural target activation from a behavioral intervention with longitudinal changes in health behavior or outcomes. This study suggests that reducing amygdala activity for threat cues during nonconscious emotion processing at an early stage through targeted intervention may improve longitudinal changes in physical activity and energy expenditure. We speculate that reductions in nonconscious threat-related amygdala reactivity may reflect an anxiolytic effect that allows for improved automatic responses to stressors or sources of negativity (e.g., sources of stress or negative stimulation in the environment related to family, work stress, safety, and schedule pressures) that might interfere with behavioral intention to be physically active (10). This supposition warrants investigation.
The results identified changes in other neural regions (i.e., functional connectivity between pgACC and anterior insula) of the negative affect circuit for sad stimuli that were predictors, albeit not mediators, of changes in caloric intake and dietary quality. These neural regions are notable for being involved in emotion processing (60). Individuals with obesity may require greater effort to inhibit craving of pleasant but unhealthy foods (e.g., high-calorie or high-fat foods), especially in an emotional context (61). In addition, the insula is directly involved in interoception and changes in insula activity could reflect actual changes in perception of internal state (such as hunger vs. fullness/satiety) (62). Although the initial PST for depression in this study may not have been specific or potent enough to change these neural targets, interventions optimized with strategies to target these neural regions may help reduce vulnerability to emotional eating in negative emotional (e.g., sad) states or hypersensitivity to interoceptive signals of hunger, thus promoting improved eating behaviors among individuals with comorbid obesity and depression. For example, prior RCTs of behavioral weight-loss or weight-gain-prevention interventions found that targeted intervention strategies (e.g., cognitive reappraisal, mindful eating, food response, and attention training) significantly reduced responses in specific neural regions (e.g., left midcingulate cortex, midinsula) (63, 64). Prior studies using functional brain imaging with food stimuli have also identified links between some of the same neural regions (e.g., amygdala, insula, ACC) and eating behaviors (e.g., overeating and food craving) and obesity or weight gain (65, 66). Future studies of behavioral interventions optimized with strategies aimed at altering these neural targets are needed to test their possible causal roles in improving eating behaviors and weight loss.
This study has several limitations. First, given that this was a mechanistic pilot study with a small sample, the findings are hypothesis generating, with more research needed to validate and extend the results. Second, although the interviewer-administered recalls of 7-d physical activity and 24-h dietary intake have been validated and widely used in behavioral studies, they are subject to recall bias and social desirability. Participants with obesity tend to overestimate their physical activity (34) and underestimate certain food types (e.g., high-fat foods, snacks) (67). One day of 24-h recalls may not adequately assess usual dietary intakes due to day-to-day variation. Future studies should use objective assessments of physical activity via accelerometry and repeated measures of dietary intake via multiple 24-h dietary recalls. Stote et al. (68) suggested more days (e.g., 3 nonconsecutive days per month over 6 mo) might be required to estimate energy intake in adults who are overweight or obese compared with those with normal weight, due to their day-to-day variation. Third, readers should be cautious about the results from multiple testing. We presented both unadjusted and adjusted P values to aid transparent interpretation and focused on results with medium or larger effect sizes and consistency across related measures. Fourth, that ENGAGE participants were a subsample of the parent RAINBOW trial may limit the net benefit of the random assignment based on Pocock's covariate-adaptive minimization, although baseline characteristics of the intervention and usual-care groups appeared balanced. Fifth, the participants were primarily women, non-Hispanic White, and college educated. Research on validation and generalizability of the current findings in independent samples with more diverse sex/gender, racial/ethnic, and socioeconomic distributions is an important next step (69). Finally, depression was not measured at 2 mo, precluding examination of whether early change in depression symptoms mediated the intervention effects on BMI and lifestyle behaviors at 12 mo, which will be addressed in our ongoing trial (69).
Despite these limitations, this pilot RCT sheds light on new hypotheses about possible neural mechanisms underlying behavioral treatment for obesity in patients with comorbid depression for testing in future trials. Moving forward, additional research is warranted to confirm the neural targets that play a causal role in the mechanism of behavior change for weight loss. This would offer a new pathway for precision treatment testing and optimization within the precision clinical trial framework (70). For instance, the identified mediators (e.g., amygdala activity and the global negative affect circuit for threat cues) or predictors (e.g., connectivity between the pgACC and anterior insula in response to sad cues) following early PST for depression, if verified in confirmatory mechanistic studies, may then be used as intermediate tailoring variables to stratify participants who need subsequent treatment adjustment for better outcomes in future trials. These neural targets could be specifically engaged with noninvasive brain stimulation (e.g., transcranial magnetic stimulation or transcranial direct current stimulation) as a way to augment the intervention for those who fail to show target engagement in the first 2 mo of intervention. Future trials may also test enhancements of the existing integrated collaborative care intervention for comorbid obesity and depression (11) with strategies that target the identified neural mediators or predictors, such as mindfulness-based emotional eating awareness training (71, 72) and acceptance and commitment therapy (73).
In conclusion, this study found that blunted amygdala reactivity and lower global scores of the negative affect circuit in response to threat stimuli following initial PST for depression mediated long-term increases in leisure-time physical activity and total energy expenditure. In addition, despite a lack of treatment effects, changes in functional connectivity between the pgACC and anterior insula engaged by sad stimuli at 2 mo predicted changes in caloric intake and dietary quality at 12 mo. These findings offer insight into possible neural mechanisms to target for behavioral weight-loss treatment enhancement in obesity with comorbid depression.
Supplementary Material
Acknowledgments
We thank the RAINBOW and ENGAGE Data and Safety Monitoring Board members (William L. Haskell, PhD, Manisha Desai, PhD, Mickey Trockel, MD, Manpreet K. Singh, MD, MS, Sandra Tsai, MD, and Alex Leow, MD, PhD) who have made substantial contributions to the conduct of the study.
The authors’ responsibilities were as follows—NL and WKL: drafted the manuscript; JM and LMW: conceived the study, led the study design and conduct, obtained funding, and have the overall responsibility for its design and conduct; PWL, JM Simmons, JM Smyth, ANG-P, JW, PS, EMV, MBS, LX, LGR, and TS: contributed to the study design and conduct; PWL, LX, ANG-P, and JW: contributed to planning the statistical analyses; and all authors: contributed to drafting the manuscript and/or participated in critical revisions for important intellectual content and read and approved the final manuscript. JM is a paid scientific consultant for Health Mentor, Inc. (San Jose, CA). LMW is on the Scientific Advisory Board for One Mind Psyberguide and the External Advisory Board for the Laureate Institute for Brain Research. OAA is the co-founder of Keywise AI and serves on the advisory boards of Blueprint Health and Embodied Labs. The other authors report no conflicts of interest.
Notes
This work is supported by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Heart, Lung, and Blood Institute grant numbers UH2HL132368 and UH3HL132368. The RAINBOW trial was supported by the National Heart, Lung, and Blood Institute of the NIH under award number R01HL119453. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the NIH. No sponsor or funding source had a role in the design or conduct of the study; collection, management, analysis, or interpretation of the data; or preparation, review, or approval of the manuscript.
Supplemental Tables 1–3 and Supplemental Methods are available from the “Supplementary Data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
LMW and JM are co–senior authors.
Abbreviations used: ACC, anterior cingulate cortex; DASH, Dietary Approach to Stop Hypertension; ENGAGE, Engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes; FDR, false discovery rate; GLB, Group Lifestyle Balance; I-CARE, Integrated Coaching for Better Mood and Weight; MET, metabolic equivalent task; NDSR, Nutrition Data System for Research; pgACC, pregenual anterior cingulate cortex; PHQ-9, 9-item Patient Health Questionnaire; PST, problem-solving therapy; RAINBOW, Research Aimed at Improving Both Mood and Weight; RCT, randomized clinical trial; ROI, region of interest; SCL-20, Symptom Checklist 20-items; sgACC, subgenual ACC; SOBC, Science of Behavior Change.
Contributor Information
Nan Lv, Institute of Health Research and Policy, University of Illinois at Chicago, Chicago, IL, USA.
Wesley K Lefferts, Institute of Health Research and Policy, University of Illinois at Chicago, Chicago, IL, USA.
Lan Xiao, Department of Epidemiology and Population Health, Stanford University, Palo Alto, CA, USA.
Andrea N Goldstein-Piekarski, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Joseph Wielgosz, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Philip W Lavori, Department of Biomedical Data Science, Stanford University, Stanford, CA, USA.
Janine M Simmons, National Institute on Aging, National Institutes of Health, Bethesda, MD, USA.
Joshua M Smyth, Departments of Biobehavioral Health and Medicine, The Pennsylvania State University, University Park, PA, USA.
Patrick Stetz, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA.
Elizabeth M Venditti, Department of Psychiatry, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Megan A Lewis, Center for Communication Science, RTI International, Seattle, WA, USA.
Lisa G Rosas, Department of Epidemiology and Population Health, Stanford University, Palo Alto, CA, USA; Department of Medicine, Stanford University, Palo Alto, CA, USA.
Mark B Snowden, Department of Psychiatry and Behavioral Sciences, University of Washington School of Medicine, Harborview Medical Center, Seattle, WA, USA.
Olusola A Ajilore, Department of Psychiatry, University of Illinois at Chicago, Chicago, IL, USA.
Trisha Suppes, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Leanne M Williams, Department of Psychiatry and Behavioral Sciences, Stanford University, Stanford, CA, USA; Sierra-Pacific Mental Illness Research, Education, and Clinical Center (MIRECC), Veterans Affairs Palo Alto Health Care System, Palo Alto, CA, USA.
Jun Ma, Institute of Health Research and Policy, University of Illinois at Chicago, Chicago, IL, USA; Department of Medicine, University of Illinois at Chicago, Chicago, IL, USA.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author only under a formal data-sharing and use agreement that provides for a commitment to the following: 1) using the data only for research purposes and not to identify any individual participant, 2) securing the data using appropriate computer technology, 3) destroying or returning the data after analyses are completed, 4) accepting reporting responsibilities, 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties, and 6) proper acknowledgement of the data resource. In addition, appropriate fees may be assessed upon mutual agreement on requests for information in a format other than that we intend to provide. We will not be responsible for providing any analytical support.
References
- 1. Pereira-Miranda E, Costa PRF, Queiroz VAO, Pereira-Santos M, Santana MLP. Overweight and obesity associated with higher depression prevalence in adults: a systematic review and meta-analysis. J Am Coll Nutr. 2017;36(3):223–33. [DOI] [PubMed] [Google Scholar]
- 2. Park BY, Hong J, Park H. Neuroimaging biomarkers to associate obesity and negative emotions. Sci Rep. 2017;7(1):7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jaworska N, Yang XR, Knott V, MacQueen G. A review of fMRI studies during visual emotive processing in major depressive disorder. World J Biol Psychiatry. 2015;16(7):448–71. [DOI] [PubMed] [Google Scholar]
- 4. Farr OM, Li CR, Mantzoros CS. Central nervous system regulation of eating: insights from human brain imaging. Metabolism. 2016;65(5):699–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Czepczor-Bernat K, Brytek-Matera A, Gramaglia C, Zeppegno P. The moderating effects of mindful eating on the relationship between emotional functioning and eating styles in overweight and obese women. Eat Weight Disord. 2019;25(4):841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Strien T. Causes of emotional eating and matched treatment of obesity. Curr Diab Rep. 2018;18(6):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Raman J, Smith E, Hay P. The clinical obesity maintenance model: an integration of psychological constructs including mood, emotional regulation, disordered overeating, habitual cluster behaviours, health literacy and cognitive function. J Obes. 2013;2013:240128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sainsbury K, Evans EH, Pedersen S, Marques MM, Teixeira PJ, Lahteenmaki L, Stubbs RJ, Heitmann BL, Sniehotta FF. Attribution of weight regain to emotional reasons amongst European adults with overweight and obesity who regained weight following a weight loss attempt. Eat Weight Disord. 2019;24(2):351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Whiteside U, Chen E, Neighbors C, Hunter D, Lo T, Larimer M. Difficulties regulating emotions: do binge eaters have fewer strategies to modulate and tolerate negative affect?. Eat Behav. 2007;8(2):162–9. [DOI] [PubMed] [Google Scholar]
- 10. Stults-Kolehmainen MA, Sinha R. The effects of stress on physical activity and exercise. Sports Medicine. 2014;44(1):81–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ma J, Rosas LG, Lv N, Xiao L, Snowden MB, Venditti EM, Lewis MA, Goldhaber-Fiebert JD, Lavori PW. Effect of integrated behavioral weight loss treatment and problem-solving therapy on body mass index and depressive symptoms among patients with obesity and depression: the RAINBOW randomized clinical trial. JAMA. 2019;321(9):869–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lv N, Xiao L, Majd M, Lavori PW, Smyth JM, Rosas LG, Venditti EM, Snowden MB, Lewis MA, Ward Eet al. . Variability in engagement and progress in efficacious integrated collaborative care for primary care patients with obesity and depression: within-treatment analysis in the RAINBOW trial. PLoS One. 2020;15(4):e0231743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rosas LG, Xiao L, Lv N, Lavori PW, Venditti EM, Snowden MB, Smyth JM, Lewis MA, Williams LM, Suppes Tet al. . Understanding mechanisms of integrated behavioral therapy for co-occurring obesity and depression in primary care: a mediation analysis in the RAINBOW trial. Transl Behav Med. 2020;11(2):382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nielsen L, Riddle M, King JW, NIHSoBCI T, Aklin WM, Chen W, Clark D, Collier E, Czajkowski S, Esposito Let al. . The NIH Science of Behavior Change Program: transforming the science through a focus on mechanisms of change. Behav Res Ther. 2018;101:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiatry. 2016;3(5):472–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Williams LM. Defining biotypes for depression and anxiety based on large-scale circuit dysfunction: a theoretical review of the evidence and future directions for clinical translation. Depress Anxiety. 2017;34(1):9–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Korgaonkar MS, Erlinger M, Breukelaar IA, Boyce P, Hazell P, Antees C, Foster S, Grieve SM, Gomes L, Williams LMet al. . Amygdala activation and connectivity to emotional processing distinguishes asymptomatic patients with bipolar disorders and unipolar depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(4):361–70. [DOI] [PubMed] [Google Scholar]
- 18. Matthews SC, Strigo IA, Simmons AN, Yang TT, Paulus MP. Decreased functional coupling of the amygdala and supragenual cingulate is related to increased depression in unmedicated individuals with current major depressive disorder. J Affect Disord. 2008;111(1):13–20. [DOI] [PubMed] [Google Scholar]
- 19. Gorka SM, Young CB, Klumpp H, Kennedy AE, Francis J, Ajilore O, Langenecker SA, Shankman SA, Craske MG, Stein MBet al. . Emotion-based brain mechanisms and predictors for SSRI and CBT treatment of anxiety and depression: a randomized trial. Neuropsychopharmacology. 2019;44(9):1639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Franklin G, Carson AJ, Welch KA. Cognitive behavioural therapy for depression: systematic review of imaging studies. Acta Neuropsychiatr. 2016;28(2):61–74. [DOI] [PubMed] [Google Scholar]
- 21. Williams LM, Korgaonkar MS, Song YC, Paton R, Eagles S, Goldstein-Piekarski A, Grieve SM, Harris AW, Usherwood T, Etkin A. Amygdala reactivity to emotional faces in the prediction of general and medication-specific responses to antidepressant treatment in the randomized iSPOT-D trial. Neuropsychopharmacology. 2015;40(10):2398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Williams LM, Pines A, Goldstein-Piekarski AN, Rosas LG, Kullar M, Sacchet MD, Gevaert O, Bailenson J, Lavori PW, Dagum Pet al. . The ENGAGE study: integrating neuroimaging, virtual reality and smartphone sensing to understand self-regulation for managing depression and obesity in a precision medicine model. Behav Res Ther. 2018;101:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ma J, Yank V, Lv N, Goldhaber-Fiebert JD, Lewis MA, Kramer MK, Snowden MB, Rosas LG, Xiao L, Blonstein AC. Research aimed at improving both mood and weight (RAINBOW) in primary care: a type 1 hybrid design randomized controlled trial. Contemp Clin Trials. 2015;43:260–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lv N, Lefferts WK, Xiao L, Goldstein-Piekarski AN, Wielgosz J, Lavori PW, Simmons JM, Smyth JM, Stetz P, Venditti EMet al. . Problem solving therapy-induced amygdala engagement mediates weight loss and lifestyle behavior change in patients with obesity and depression: a randomized controlled trial. Research Square. 2020. doi: 10.21203/rs.3.rs-53210/v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xiao L, Huang Q, Yank V, Ma J. An easily accessible Web-based minimization random allocation system for clinical trials. J Med Internet Res. 2013;15(7):e139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103–15. [PubMed] [Google Scholar]
- 27. Kramer MK, Kriska AM, Venditti EM, Miller RG, Brooks MM, Burke LE, Siminerio LM, Solano FX, Orchard TJ. Translating the Diabetes Prevention Program: a comprehensive model for prevention training and program delivery. Am J Prev Med. 2009;37(6):505–11. [DOI] [PubMed] [Google Scholar]
- 28. Ciechanowski P, Wagner E, Schmaling K, Schwartz S, Williams B, Diehr P, Kulzer J, Gray S, Collier C, LoGerfo J. Community-integrated home-based depression treatment in older adults: a randomized controlled trial. JAMA. 2004;291(13):1569–77. [DOI] [PubMed] [Google Scholar]
- 29. Ciechanowski P, Chaytor N, Miller J, Fraser R, Russo J, Unutzer J, Gilliam F. PEARLS depression treatment for individuals with epilepsy: a randomized controlled trial. Epilepsy Behav. 2010;19(3):225–31. [DOI] [PubMed] [Google Scholar]
- 30. Measures from the PhenX Toolkit version May 5, Version 38.0. [Internet]. Available from: www.phenxtoolkit.org (accessed 24 May 2021). [Google Scholar]
- 31. Blair SN, Haskell WL, Ho P, Paffenbarger RS Jr, Vranizan KM, Farquhar JW, Wood PD. Assessment of habitual physical activity by a seven-day recall in a community survey and controlled experiments. Am J Epidemiol. 1985;122(5):794–804. [DOI] [PubMed] [Google Scholar]
- 32. Conway JM, Ingwersen LA, Vinyard BT, Moshfegh AJ. Effectiveness of the US Department of Agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171–8. [DOI] [PubMed] [Google Scholar]
- 33. Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104(4):595–603. [DOI] [PubMed] [Google Scholar]
- 34. Washburn RA, Jacobsen DJ, Sonko BJ, Hill JO, Donnelly JE. The validity of the Stanford Seven-Day Physical Activity Recall in young adults. Med Sci Sports Exerc. 2003;35(8):1374–80. [DOI] [PubMed] [Google Scholar]
- 35. Moshfegh AJ, Rhodes DG, Baer DJ, Murayi T, Clemens JC, Rumpler WV, Paul DR, Sebastian RS, Kuczynski KJ, Ingwersen LAet al. . The US Department of Agriculture Automated Multiple-Pass Method reduces bias in the collection of energy intakes. Am J Clin Nutr. 2008;88(2):324–32. [DOI] [PubMed] [Google Scholar]
- 36. Sallis JF, Haskell WL, Wood PD, Fortmann SP, Rogers T, Blair SN, Paffenbarger RS Jr. Physical activity assessment methodology in the Five-City Project. Am J Epidemiol. 1985;121(1):91–106. [DOI] [PubMed] [Google Scholar]
- 37. Wilson PW, Paffenbarger RS, Morris JN, Havlik RJ. Assessment methods for physical activity and physical fitness in population studies: report of a NHLBI workshop. Am Heart J. 1986;111(6):1177–92. [DOI] [PubMed] [Google Scholar]
- 38. Mellen PB, Gao SK, Vitolins MZ, Goff DCJr. Deteriorating dietary habits among adults with hypertension: DASH dietary accordance, NHANES 1988–1994 and 1999–2004. Arch Intern Med. 2008;168(3):308–14. [DOI] [PubMed] [Google Scholar]
- 39. Lovden M, Wenger E, Martensson J, Lindenberger U, Backman L. Structural brain plasticity in adult learning and development. Neurosci Biobehav Rev. 2013;37(9 Pt B):2296–310. [DOI] [PubMed] [Google Scholar]
- 40. Korgaonkar MS, Grieve SM, Etkin A, Koslow SH, Williams LM. Using standardized fMRI protocols to identify patterns of prefrontal circuit dysregulation that are common and specific to cognitive and emotional tasks in major depressive disorder: first wave results from the iSPOT-D study. Neuropsychopharmacology. 2013;38(5):863–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goldstein-Piekarski AN, Ball TM, Samara Z, Staveland BR, Keller AS, Fleming SL, Grisanzio KA, Holt-Gosselin B, Stetz P, Ma Jet al. . Mapping neural circuit biotypes to symptoms. and behavioral dimensions of depression and anxiety. Biol Psychiatry. Published online July 11, 2021. doi: https://doi.org/10.1016/j.biopsych.2021.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Goldstein-Piekarski AN, Korgaonkar MS, Green E, Suppes T, Schatzberg AF, Hastie T, Nemeroff CB, Williams LM. Human amygdala engagement moderated by early life stress exposure is a biobehavioral target for predicting recovery on antidepressants. Proc Natl Acad Sci. 2016;113(42):11955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–29. [DOI] [PubMed] [Google Scholar]
- 44. Ball TM, Goldstein-Piekarski AN, Gatt JM, Williams LM. Quantifying person-level brain network functioning to facilitate clinical translation. Transl Psychiatry. 2017;7(10):e1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Goldstein-Piekarski AN, Wielgosz J, Xiao L, Stetz P, Correa CG, Chang SE, Lv N, Rosas LG, Lavori PW, Snowden MBet al. . Early changes in neural circuit function engaged by negative emotion and modified by behavioural intervention are associated with depression and problem-solving outcomes: a report from the ENGAGE randomized controlled trial. EBioMedicine. 2021;67:103387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Arch Gen Psychiatry. 2002;59(10):877–83. [DOI] [PubMed] [Google Scholar]
- 47. Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Stat. 2001;29:1165–88. [Google Scholar]
- 48. Wasserstein RL, Lazar NA. The ASA statement on p-values: context, process, and purpose. Am Statist. 2016;70(2):129–33. [Google Scholar]
- 49. Sorkin JD, Manary M, Smeets PAM, MacFarlane AJ, Astrup A, Prigeon RL, Hogans BB, Odle J, Davis TA, Tucker KLet al. . A guide for authors and readers of the American Society for Nutrition journals on the proper use of P values and strategies that promote transparency and improve research reproducibility. Am J Clin Nutr. Published online July 13, 2021. doi: 10.1093/ajcn/nqab223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fritz MS, Mackinnon DP. Required sample size to detect the mediated effect. Psychol Sci. 2007;18(3):233–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weng TB, Pierce GL, Darling WG, Falk D, Magnotta VA, Voss MW. The acute effects of aerobic exercise on the functional connectivity of human brain networks. Brain Plasticity. 2017;2(2):171–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Z, Wu Y, Li L, Guo X. Functional connectivity within the executive control network mediates the effects of long-term tai chi exercise on elders' emotion regulation. Front Aging Neurosci. 2018;10:315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cornier MA, Melanson EL, Salzberg AK, Bechtell JL, Tregellas JR. The effects of exercise on the neuronal response to food cues. Physiol Behav. 2012;105(4):1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McFadden KL, Cornier MA, Melanson EL, Bechtell JL, Tregellas JR. Effects of exercise on resting-state default mode and salience network activity in overweight/obese adults. Neuroreport. 2013;24(15):866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mokhtari F, Rejeski WJ, Zhu Y, Wu G, Simpson SL, Burdette JH, Laurienti PJ. Dynamic fMRI networks predict success in a behavioral weight loss program among older adults. Neuroimage. 2018;173:421–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gallen CL, D'Esposito M. Brain modularity: a biomarker of intervention-related plasticity. Trends Cogn Sci. 2019;23(4):293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Baniqued PL, Gallen CL, Voss MW, Burzynska AZ, Wong CN, Cooke GE, Duffy K, Fanning J, Ehlers DK, Salerno EAet al. . Brain network modularity predicts exercise-related executive function gains in older adults. Front Aging Neurosci. 2017;9:426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gallen CL, Baniqued PL, Chapman SB, Aslan S, Keebler M, Didehbani N, D'Esposito M. Modular brain network organization predicts response to cognitive training in older adults. PLoS One. 2016;11(12):e0169015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Arnemann KL, Chen AJ, Novakovic-Agopian T, Gratton C, Nomura EM, D'Esposito M. Functional brain network modularity predicts response to cognitive training after brain injury. Neurology. 2015;84(15):1568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nomi JS, Schettini E, Broce I, Dick AS, Uddin LQ. Structural connections of functionally defined human insular subdivisions. Cereb Cortex. 2018;28(10):3445–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wang GJ, Shokri Kojori E, Yuan K, Wiers CE, Manza P, Wong CT, Fowler JS, Volkow ND. Inhibition of food craving is a metabolically active process in the brain in obese men. Int J Obes. 2020;44(3):590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Simmons WK, DeVille DC. Interoceptive contributions to healthy eating and obesity. Curr Opin Psychol. 2017;17:106–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stice E, Yokum S, Burger K, Rohde P, Shaw H, Gau JM. A pilot randomized trial of a cognitive reappraisal obesity prevention program. Physiol Behav. 2015;138:124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Stice E, Yokum S, Veling H, Kemps E, Lawrence NS. Pilot test of a novel food response and attention training treatment for obesity: brain imaging data suggest actions shape valuation. Behav Res Ther. 2017;94:60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Monteleone AM, Castellini G, Volpe U, Ricca V, Lelli L, Monteleone P, Maj M. Neuroendocrinology and brain imaging of reward in eating disorders: a possible key to the treatment of anorexia nervosa and bulimia nervosa. Prog Neuropsychopharmacol Biol Psychiatry. 2018;80(Pt B):132–42. [DOI] [PubMed] [Google Scholar]
- 66. Stice E, Figlewicz DP, Gosnell BA, Levine AS, Pratt WE. The contribution of brain reward circuits to the obesity epidemic. Neurosci Biobehav Rev. 2013;37(9 Pt A):2047–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lissner L. Measuring food intake in studies of obesity. Public Health Nutr. 2002;5(6a):889–92. [DOI] [PubMed] [Google Scholar]
- 68. Stote KS, Radecki SV, Moshfegh AJ, Ingwersen LA, Baer DJ. The number of 24 h dietary recalls using the US Department of Agriculture's automated multiple-pass method required to estimate nutrient intake in overweight and obese adults. Public Health Nutr. 2011;14(10):1736–42. [DOI] [PubMed] [Google Scholar]
- 69. Lv N, Ajilore OA, Ronneberg CR, Venditti EM, Snowden MB, Lavori PW, Xiao L, Goldstein-Piekarski AN, Wielgosz J, Wittels NEet al. . The ENGAGE-2 study: engaging self-regulation targets to understand the mechanisms of behavior change and improve mood and weight outcomes in a randomized controlled trial (phase 2). Contemp Clin Trials. 2020;95:106072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lenze EJ, Nicol GE, Barbour DL, Kannampallil T, Wong AWK, Piccirillo J, Drysdale AT, Sylvester CM, Haddad R, Miller JPet al. . Precision clinical trials: a framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci. 2021;46(1):E97–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lattimore P. Mindfulness-based emotional eating awareness training: taking the emotional out of eating. Eat Weight Disord. 2019;25(3):649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katterman SN, Kleinman BM, Hood MM, Nackers LM, Corsica JA. Mindfulness meditation as an intervention for binge eating, emotional eating, and weight loss: a systematic review. Eat Behav. 2014;15(2):197–204. [DOI] [PubMed] [Google Scholar]
- 73. Jarvela-Reijonen E, Karhunen L, Sairanen E, Muotka J, Lindroos S, Laitinen J, Puttonen S, Peuhkuri K, Hallikainen M, Pihlajamaki Jet al. . The effects of acceptance and commitment therapy on eating behavior and diet delivered through face-to-face contact and a mobile app: a randomized controlled trial. Int J Behav Nutr Phys Act. 2018;15(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author only under a formal data-sharing and use agreement that provides for a commitment to the following: 1) using the data only for research purposes and not to identify any individual participant, 2) securing the data using appropriate computer technology, 3) destroying or returning the data after analyses are completed, 4) accepting reporting responsibilities, 5) abiding by restrictions on redistribution of the data for commercial purposes or to third parties, and 6) proper acknowledgement of the data resource. In addition, appropriate fees may be assessed upon mutual agreement on requests for information in a format other than that we intend to provide. We will not be responsible for providing any analytical support.