Table 1.
Inhibitory activities of 9,10-dihydrophenanthrene derivatives against SARS-CoV-2 3CLpro.
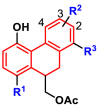 | ||||
|---|---|---|---|---|
| Compds | R1 | R2 | R3 | IC50 ± SD (μM)a |
| A1 | CH3 | H | 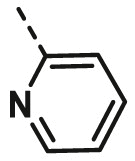 |
61.15 ± 3.60 |
| A2 | C2H5 | H | 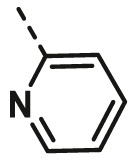 |
33.06 ± 8.58 |
| A3 | isopropyl | H | 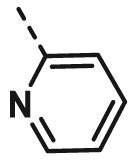 |
29.46 ± 1.64 |
| A4 | cyclohexyl | H | 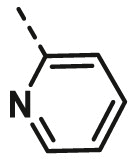 |
9.06 ± 0.34 |
| A5 | 4-Br Phenyl | H | 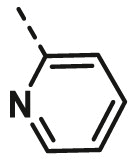 |
6.44 ± 0.64 |
| A6 | 4-CN Phenyl | H | 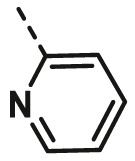 |
19.32 ± 1.92 |
| A7 | benzyl | H | 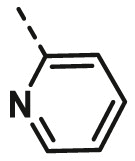 |
11.39 ± 0.64 |
| A8 | CH3 | 4-CH3 | 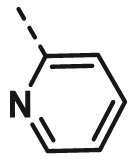 |
>100 |
| A9 | CH3 | 4-OCH3 | 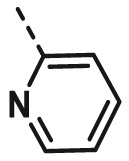 |
>100 |
| A10 | CH3 | 4-F | 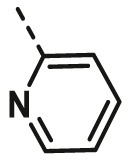 |
>100 |
| A11 | CH3 | 4-Cl | 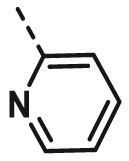 |
85.58 ± 2.36 |
| A12 | CH3 | 4-Br | 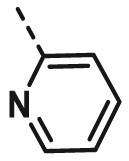 |
>100 |
| A13 | CH3 | 3-CH3 | 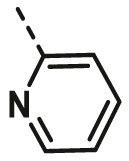 |
57.67 ± 2.78 |
| A14 | CH3 | 2-CH3 | 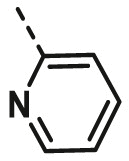 |
53.81 ± 8.49 |
| A15 | CH3 | H | 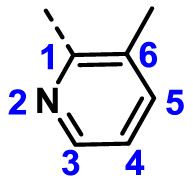 |
58.91 ± 4.58 |
| A16 | CH3 | H | 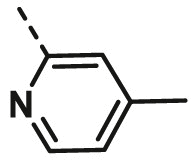 |
68.16 ± 2.64 |
| A17 | CH3 | H | 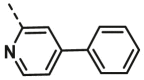 |
5.65 ± 1.40 |
| A18 | CH3 | H | 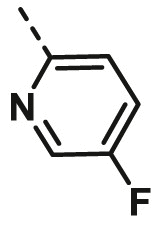 |
66.80 ± 2.74 |
| A19 | CH3 | H | 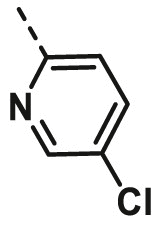 |
35.77 ± 2.96 |
| A20 | CH3 | H | 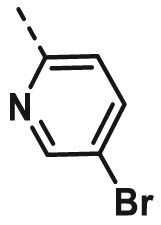 |
18.50 ± 0.76 |
| A21 | CH3 | H | 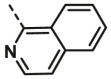 |
14.17 ± 1.30 |
| A22 | CH3 | H | 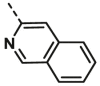 |
11.97 ± 0.68 |
| A23 | CH3 | H | 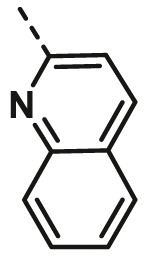 |
13.82 ± 0.90 |
| A24 | CH3 | H | 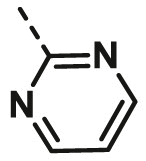 |
>100 |
| A25 | CH3 | H | 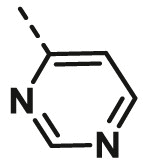 |
>100 |
| A26 | CH3 | H | 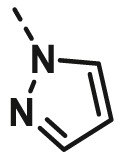 |
>100 |
| A27 | CH3 | H | 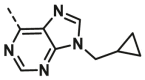 |
>100 |
| A28 | CH3 | H | 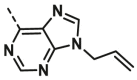 |
>100 |
| disulfiram | – | – | – | 1.04 ± 0.03 |
The inhibitory effects of these compounds against SARS-CoV-2 3CLpro were determined by the FRET assay. The data are the mean ± SD from at least three independent experiments.
