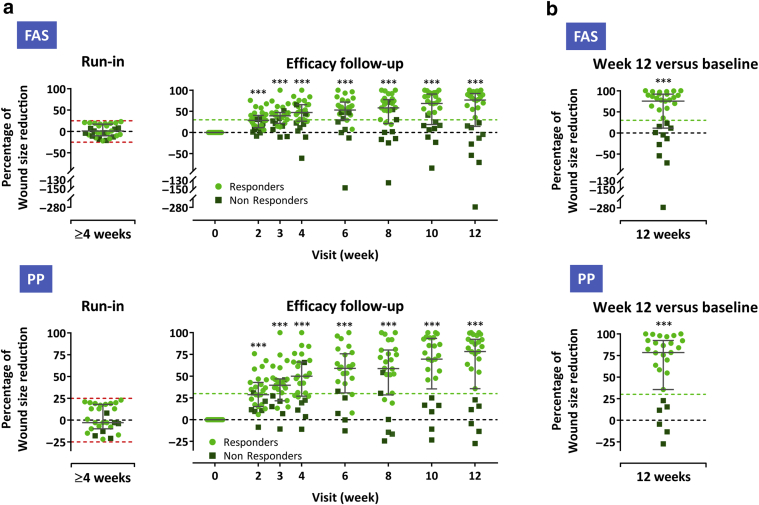
Figure 2.
Wound healing progress during the run-in and the treatment and efficacy follow-up period. (a) Percent wound size reduction during ≥4-week screening (run-in) period and during 12-week treatment/efficacy follow-up (presented as a reduction from baseline) in the FAS (n = 31) (upper panel) and PP (n = 27) (lower panel). (b) Percent wound size reduction from baseline at week 12 (last observation carried forward) in the FAS (upper panel) and PP (lower panel). Subjects whose wounds diminished or enlarged by >25% (dashed red lines) during the screening period did not qualify for study treatment. Subjects who had wound size reductions of at least 30% from baseline (indicated by light green dashed lines) at week 12 were considered responders. Error bars indicate median and interquartile range; ∗∗∗P < 0.001 versus baseline, two-sided Wilcoxon signed-rank test. FAS, full analysis set; PP, per-protocol set.