Figure 3: Effect of NIR laser and chemical adjuvants on skin tissue.
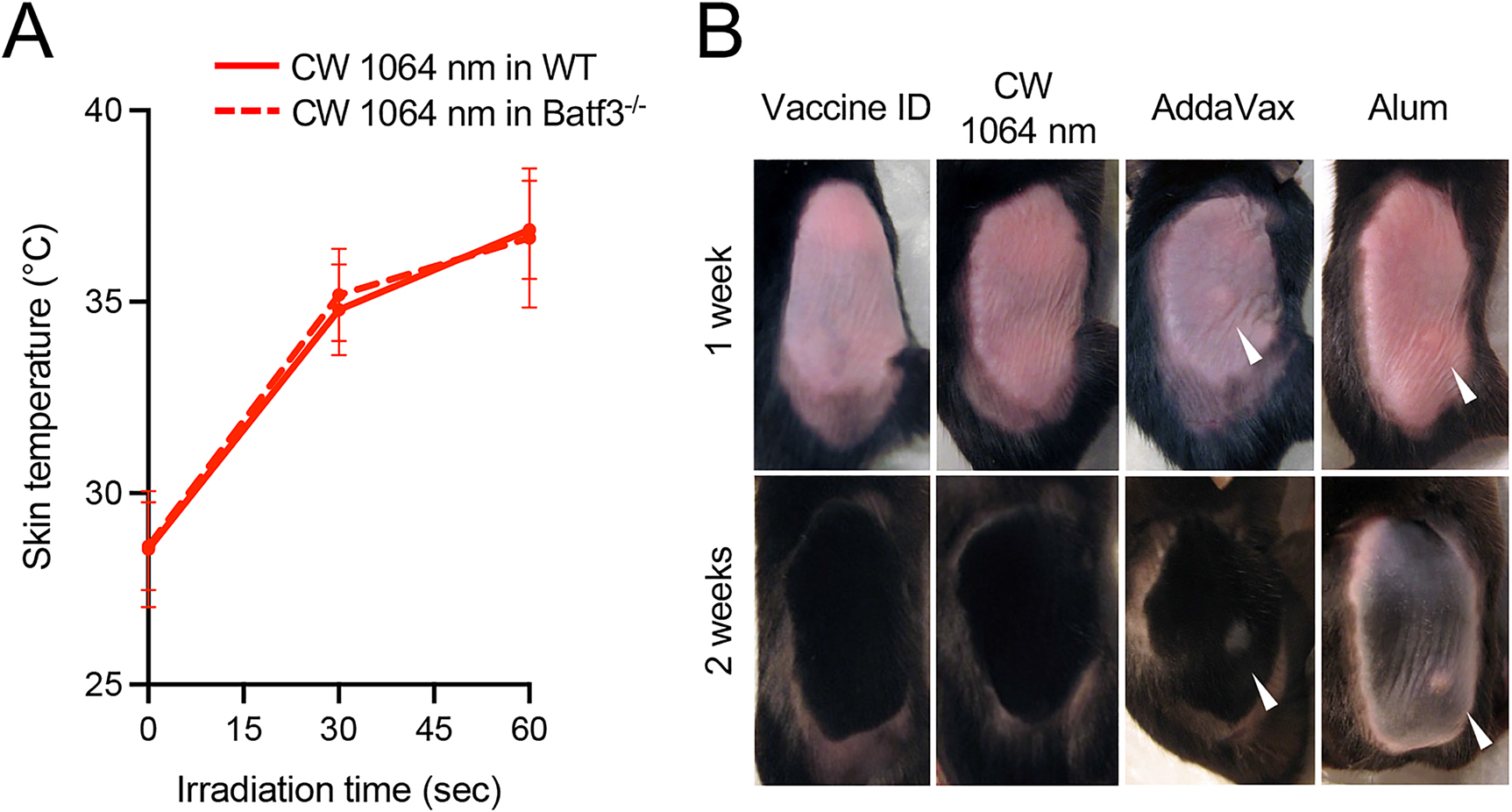
(A) Dose–temperature responses of the CW 1064 nm laser in mouse skin. Error bars show means ± s.d. n = 20, 8 for vaccine ID + NIR laser in WT and vaccine ID + NIR laser in Batf3−/− groups, respectively. (B) Images of the back of mice for visual inspection at 1 and 2 weeks after the application of NIR laser or chemical adjuvant. WT and Batf3−/− mice were vaccinated ID with 1 μg of inactivated influenza virus (A/PR/8/34) with or without the NIR laser exposure or alum or AddaVax adjuvant. n = 6, 7, 3, 5 for vaccine ID only in WT, vaccine ID + NIR laser in WT, vaccine + Alum ID in WT, vaccine + AddaVax ID in WT groups, respectively. Representative images for each group are presented.
