Abstract
In patients with nonpalpable early-stage breast cancer eligible for breast conservation surgery (BCS), wire-guided localization (WGL) is widely accepted as a standard technique for preoperative image-guided lesion localization. In preparation for this procedure, lesion location, size, type and configuration play important roles in preoperative localization planning. Successful preoperative planning requires review of all pertinent imaging studies, imaging reports and pathology reports, with special attention to pre- and post-biopsy imaging and evaluation of the targeted lesions and the type and the position of the marker clips. Preoperative communication with the surgeon is key in the planning process to ensure that clarity in localization objectives are reached in complex cases. This pictorial essay will provide a methodical, step by step approach to planning successful image-guided preoperative needle localizations. These steps include selection of the imaging modality, the equipment, the procedure and intraoperative specimen radiography. The case-based review will also include key steps and considerations during the planning stage, the procedure stage, and the post-procedure stage. These same techniques can also be applied to newer, non-wire image-guided breast localization techniques now available for widespread commercial use.
Keywords: Wire-guided localization, Needle localization, Ultrasound-guided needle localization, Mammography-guided needle localization, Specimen radiography
Introduction
The number of nonpalpable early-stage breast cancers in the United States continues to increase each year. A significant proportion of this increase can be attributed to the improved utilization of and the ongoing technical advances achieved by screening mammography [1,2]. Breast-conservation therapy (BCS) has become the preferred treatment for patients with early-stage breast cancer, as randomized trials have shown that BCS with adjuvant radiotherapy with or without chemotherapy provides equivalent survival rates when compared with mastectomy in similar patient populations [3].
In patients with nonpalpable early-stage breast cancer eligible for breast conservation surgery (BCS), wire-guided localization (WGL) is widely accepted as a standard technique for preoperative image-guided lesion localization. More recently, new non-wire guided techniques have become available for preoperative localization. These include the use of radioactive iodine-131 seeds, electromagnetic radar reflectors, radiofrequency identification, and magnetic seed technology [4]. The general concepts and considerations covered in this pictorial essay can be applied to most image-guided breast localizations regardless of the device used.
This pictorial essay will provide a methodical, step by step, approach to planning a successful image-guided preoperative needle localization. The case-based review will include key steps and considerations during the planning stage, the procedure stage, and the post-procedure stage. We aim to engage practicing radiologists who can use this methodology to improve preoperative localization planning and drive local changes that can improve patient care.
Step 1: Selection of the Imaging Modality
In preparation for the procedure, lesion size, type and configuration play an important role in preoperative localization planning. Successful preoperative planning also requires review of all pertinent imaging studies, imaging reports and pathology reports with special attention to pre- and post-biopsy imaging and evaluation of the targeted lesions and the type and the position of the marker clips. Preoperative communication with the surgeon is key in the planning process to ensure that clarity in the localization objectives are reached in complex cases.
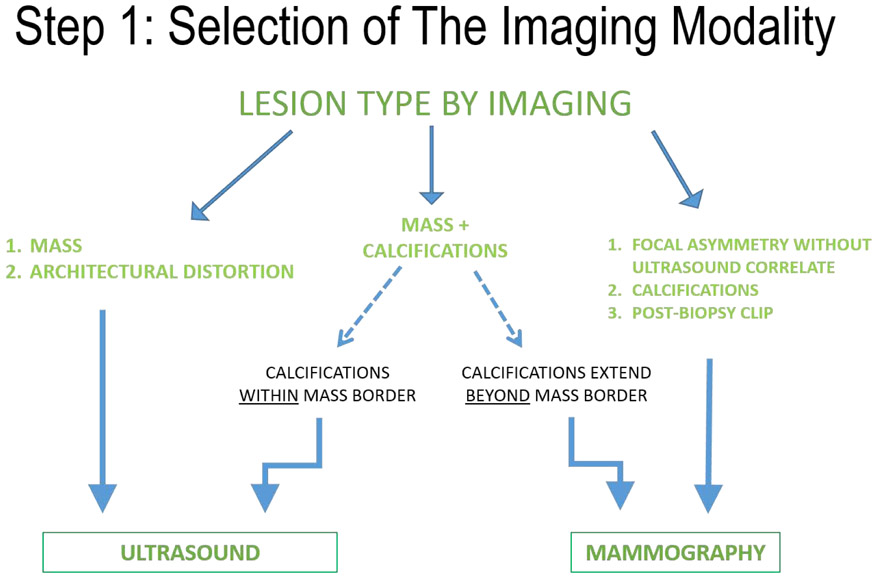
There are a number of factors that should be considered when determining the modality of choice for preoperative localization (Figure 1). When reviewing the case, all relevant imaging studies should be readily available for review. The radiologist should note the association of the targeted lesion with calcifications, the lesion span, a prior history of neoadjuvant chemotherapy, as well as the location of the post-biopsy clip(s) relative to the targeted lesion(s). If there is a history of neoadjuvant therapy, review of multimodality pre-therapy imaging is key in determining the presumed tumor bed (footprint of the residual disease). While MRI is usually the imaging modality of choice to monitor neoadjuvant therapy response, localization under mammography or ultrasound guidance is usually preferred. In these cases, using mammographic landmarks and the location of the clip markers with respect to residual enhancement helps to determine the scope of the pre-operative localization and guide surgical excision. In a small study (n = 29) of cancers that initially presented as microcalcifications treated with neoadjuvant chemotherapy, residual microcalcifications were associated with residual tumor in 55.2% of cases [5]. Though the actual extent of residual calcifications has been in some situations shown not to correlate histopathologically with residual disease, complete excision of tumor bed calcifications remains standard practice until additional studies better define which histologic and biologic patient subpopulations may be candidates for more conservative treatment. Therefore, the localized tumor bed should include any visible calcifications in these cases regardless of the degree of response on ultrasound or MRI.
FIGURE 1.
The above factors should be considered when determining the modality of choice for preoperative breast localization.
Contrast-enhanced spectral mammography [CESM] is increasingly used in breast cancer extent of disease evaluation [6]. For additional lesions identified on CESM alone, the patients are usually referred to breast MRI for biopsy or needle localization. If a contraindication to MRI exists, low-energy CESM can be used to localize the area using mammographic landmarks. CT guided localization may be performed on biopsy proven metastatic axillary nodes with metallic clips which are not seen on ultrasound following neoadjuvant therapy.
In a small population of patients with nipple discharge, intraductal lesions identified on ductography may warrant ductography-guided needle localization to help ensure that the correct discharging duct is removed. In these cases, methylene blue is also frequently injected within the discharging duct to enhance the visibility of the ductal system intraoperatively.
Having the following general rules can aid in the selection of the imaging modality. If the lesion is readily identified on ultrasound, consider ultrasound first due to the ease of patient positioning and targeting. If one or more of the lesions are better visualized on mammography, consider mammographic guidance for targeting all lesions. One may need to consider tomosynthesis or stereotactic targeting for lesions seen on only one view. Figure 1 helps to delineate these considerations pictorially.
Step 2: The Equipment
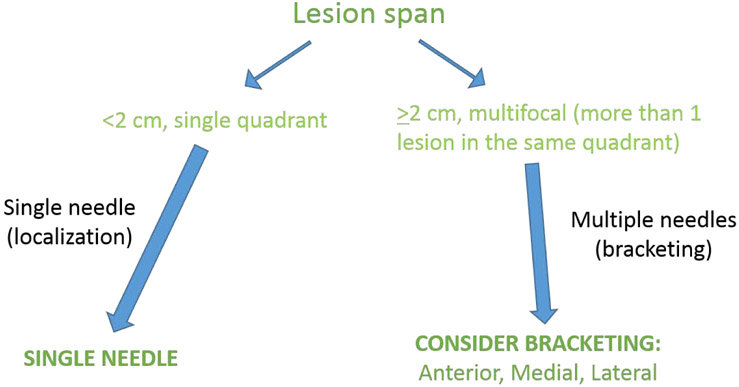
Just as important as determining the imaging modality for localization, the radiologist needs to ensure that the proper equipment is available for the localization procedure. At a minimum, the radiologist will need local anesthetic (commonly 1% of 2% lidocaine HCL at a 10mg/mL concentration), a skin sterilization agent such as chlorohexidine or iodine, a 5-10 mL syringe with a needle for anesthetizing the skin, and a localization needle with a pre-loaded wire (Figure 2A).8.5% sodium bicarbonate can also be used as a buffering agent to decrease the pain of injection. There are a number of factors that also play a role in determining the type and the number of needles for localization, as depicted in Figures 2B and 2C. The Kopans wire is the most widely used hook wire in practice, ranging in size from 3-15 cm. MRI compatible wires are also available. Similarly, the Hawkins wire is a braided hook wire with common use in women with dense breasts and ranges in size from 3 to 12.5 cm. The Homer or “J” shaped memory wire ranges in size from 3-12.5 cm and is best known for its retractibility after placement.
FIGURE 2A.
The most commonly used ancillary equipment for image-guided breast localizations.
FIGURE 2B.
There are several options to consider when selecting a pre-loaded needle for wire localization, as depicted above.
FIGURE 2C.
The number of needles required for successful wire localization depends on careful assessment of the size and the extent of the disease in the breast.
When selecting wire length for a mammography-guided needle localization, the location of the lesion helps best determine the approach and the wire length. The shortest distance from the overlying skin to the lesion should be the primary consideration, provided that the approach does not result in the wire traversing other malignant or suspicious lesions. In instances where this is a potential scenario, an alternative approach should be selected. As a general rule, the length of the needle should always exceed the distance from the lesion to the overlying skin using the designated approach.
Step 3: The Procedure Itself
The steps required for localization are largely dependent on the modality that is being used. Both mammography and ultrasound account for the majority of wire-guided localizations as lesions are most readily detected, biopsied and diagnosed using these two modalities. Larger lesions, calcifications and calcifications with spans exceeding the mass borders frequently require mammographic guidance and multiple needles for bracketing. When using multiple needles to bracket lesions, the anterior, medial and lateral borders should be prioritized. In many cases, the pectoralis fascia can be used as an anatomic border and does not require a separate needle. Communication with the surgeon is key in decision making for larger lesions and complex cases.
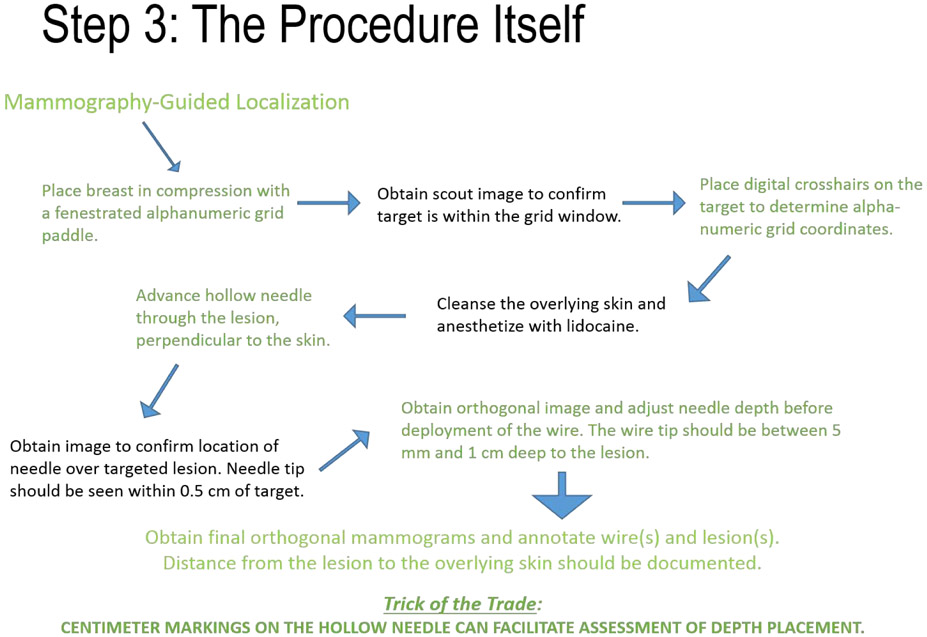
During the placement of a non-retractable wire, the radiologist has the option of leaving the wire pre-loaded in the needle hub or unsheathing it at deployment. The radiologist should discuss these options with the surgeon pre-procedurally. Figures 3A and 3B depict the stepwise procedural components for mammography-guided and ultrasound-guided localization, respectively.
FIGURE 3A.
Procedural steps in mammography-guided wire localization.
FIGURE 3B.
Procedural steps in mammography-guided wire localization.
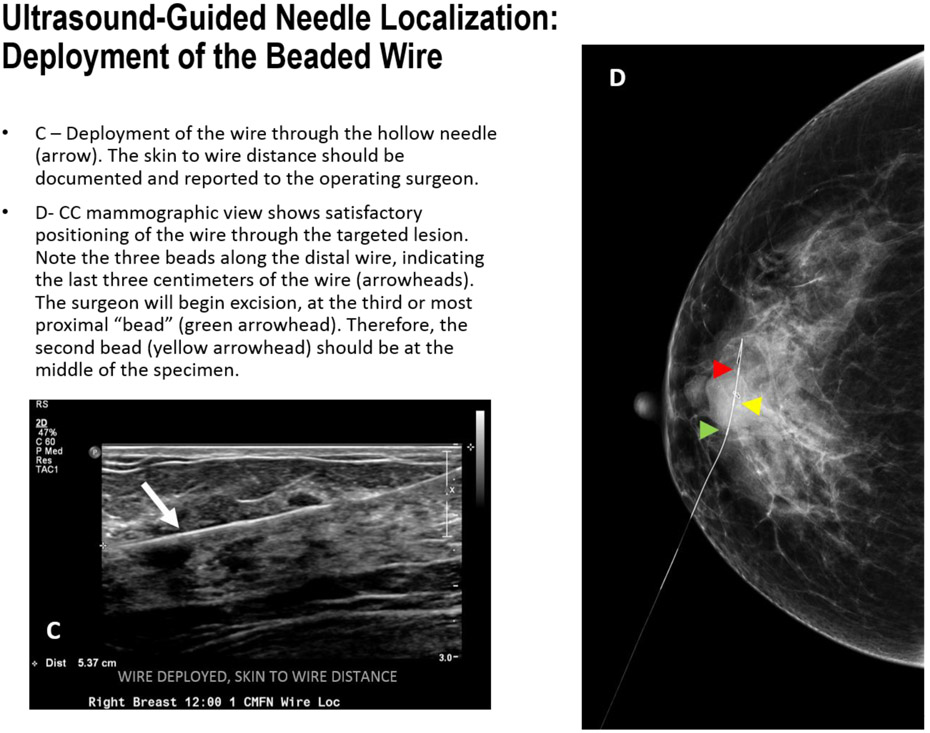
Some facilities offer a “beaded” wire containing three beads or a thickened portion that demarcates the last three centimeters. The surgeon can use this as an indication to start cutting. Therefore, the wire should be placed roughly 1 cm past the targeted lesion to ensure that the lesion is centered at the second bead which should reside in the middle of the excised specimen.
Step 4: Specimen Radiography
Intraoperative margin assessment requires multidisciplinary collaboration between surgeons, pathologists and radiologists. Surgeons may use differing methods for specimen orientation, which may involve denotation with surgical clips, long and short suture lengths and charms. It is important that the radiologist familiarize themselves with the surgeon’s practice.
Many surgical practices now utilize specimen radiography units within the operating room to eliminate the time involved in transporting the specimen to the mammography suite. A minimum of two orthogonal images should be obtained to improve margin assessment. At our institution, the en-bloc specimen is radiographed to identify the targeted lesion and then inked to identify orientation of the superior, inferior, lateral, medial, anterior and posterior margins. The specimen is then sectioned into 3 – 5 mm slices by the pathologist. At our institution, the radiologist reviews the images to verify complete local excision of the lesion and the location of the lesion relative to the margins and discusses the findings with the surgeon and/or the pathologist.
In 2014, the American Society for Radiation Oncology (ASTRO) and the Society of Surgical Oncology (SSO) issued a consensus guideline defining an adequate margin for invasive breast cancer as the absence of tumor at the actual edge of the resected tissue (“no ink on tumor”) [7]. Though not widely utilized, several studies have shown that intraoperative margin assessment by gross pathological examination and sliced specimen radiography significantly impacts intraoperative decision making, and excision of further tissue on the basis of intraoperative assessment results in a substantial decrease in re-excision surgeries for margin control. At our institution, multidisciplinary intraoperative specimen radiography assessment has assisted in reducing re-excision rates after breast conservation surgery from 21% to 7% [8].
Conclusion
In summary, careful review of pre-operative imaging is warranted to determine the optimal modality for a breast localization procedures. Pre- and post-localization communication with the surgeon is key to maximizing chances of success and negative final margins. Post-localization, the approach and the final position of the localizing needle(s) should be relayed to the surgeon through well-annotated mammograms as well as a diagram depicting the needle positions.
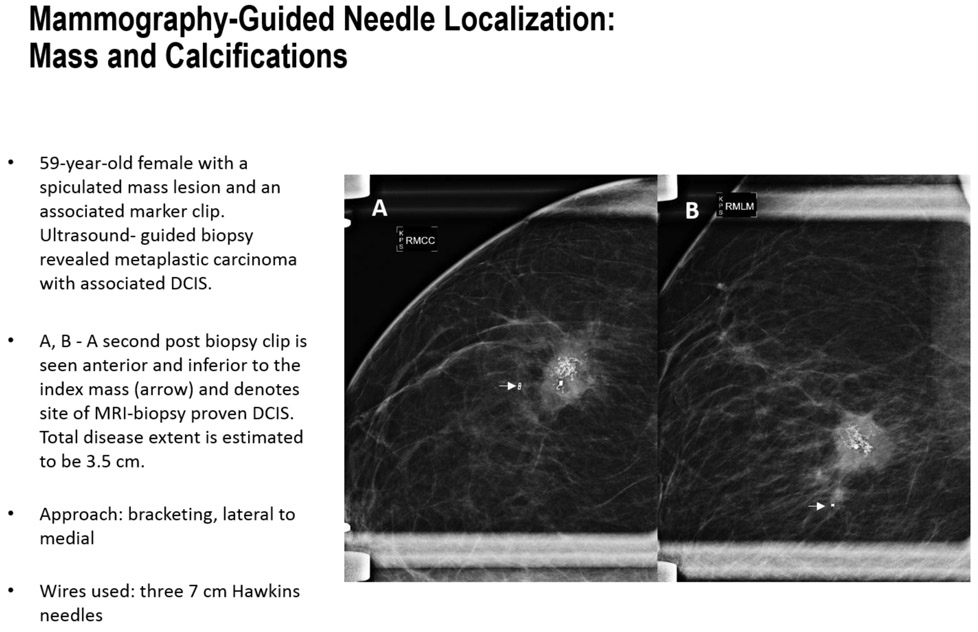
FIGURES 4A-B.
Step by step mammography-guided localization of a mass and calcifications.
FIGURES 4C-E.
Step by step mammography-guided localization of mass and calcifications.
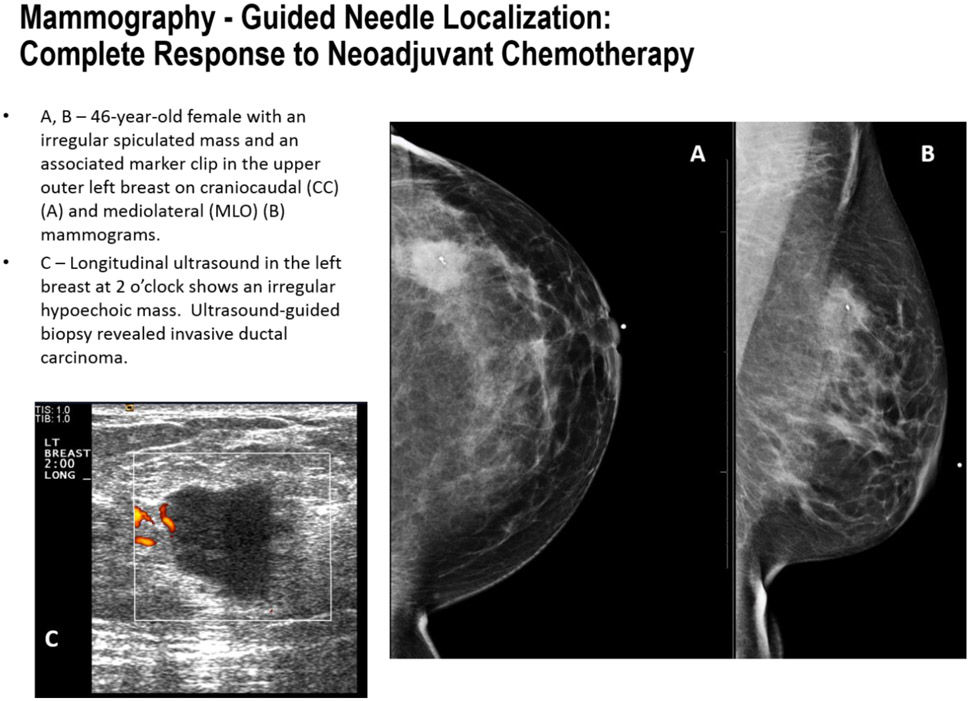
FIGURE 5A-C.
Mammography guided localization in a patient with complete response to neoadjuvant chemotherapy.
FIGURE 5D-F.
Mammography-guided localization in a patient with complete response to neoadjuvant chemotherapy.
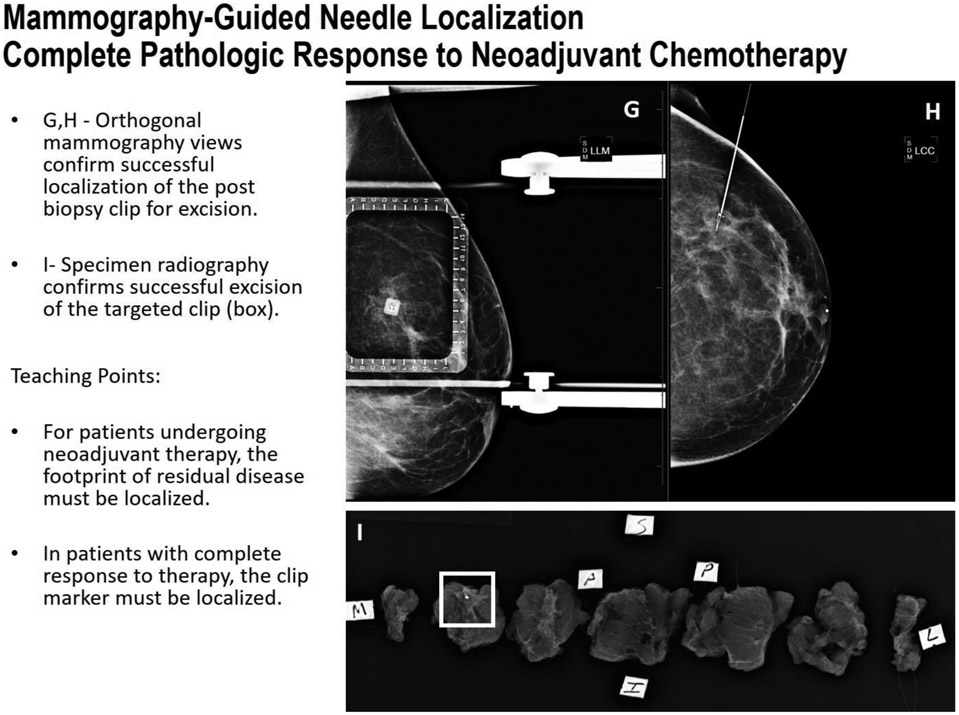
FIGURE 5G-I.
Mammography-guided localization in a patient with complete response to neoadjuvant chemotherapy.
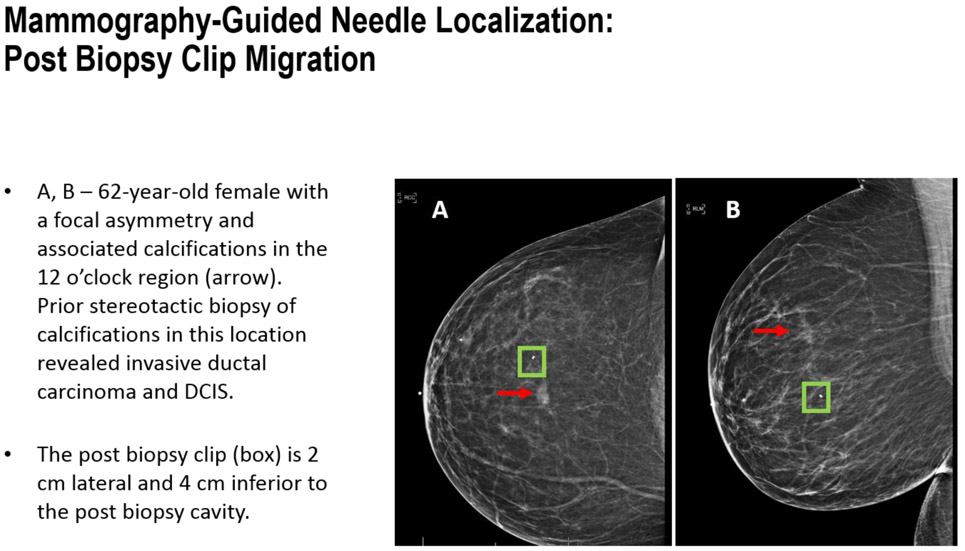
FIGURE 6A-B.
Mammography-Guided Needle Localization: Post Biopsy Clip Migration
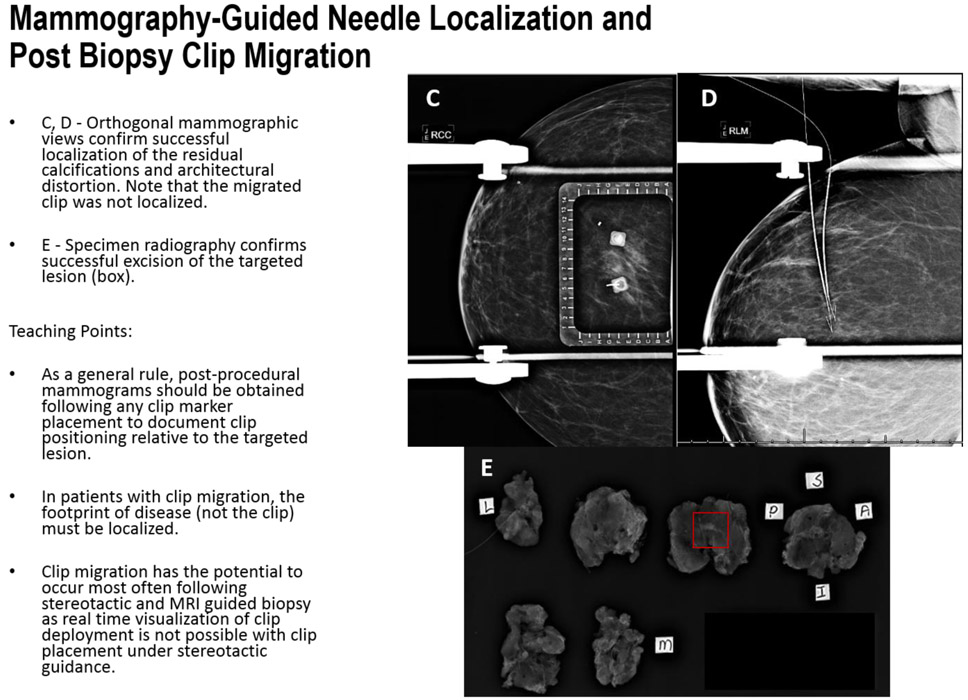
FIGURE 6C-E.
Mammography-Guided Needle Localization: Post Biopsy Clip Migration
FIGURE 7A-C.
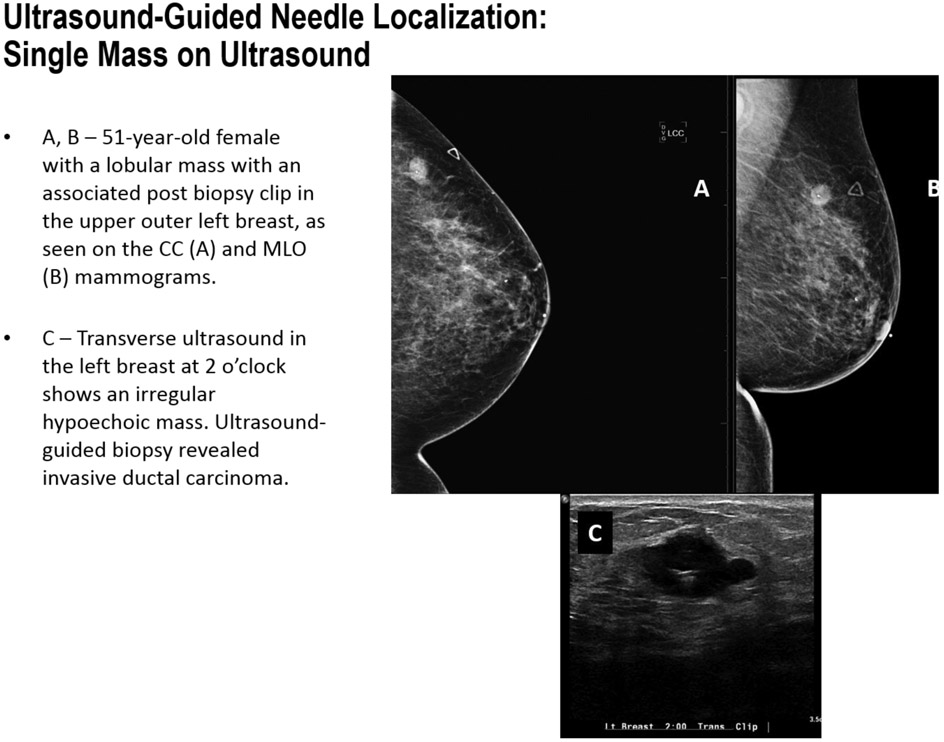
Ultrasound-Guided Needle Localization: Single Mass on Ultrasound
FIGURE 7D.
Ultrasound-Guided Needle Localization: Single Mass on Ultrasound
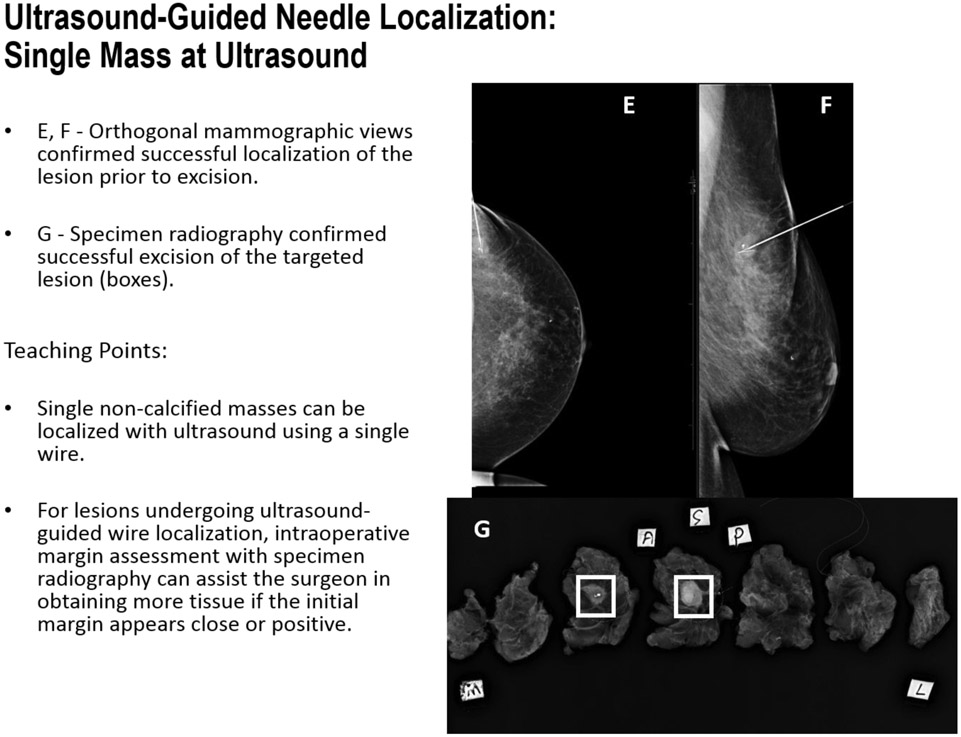
FIGURE 7E-G.
Ultrasound-Guided Needle Localization: Single Mass on Ultrasound
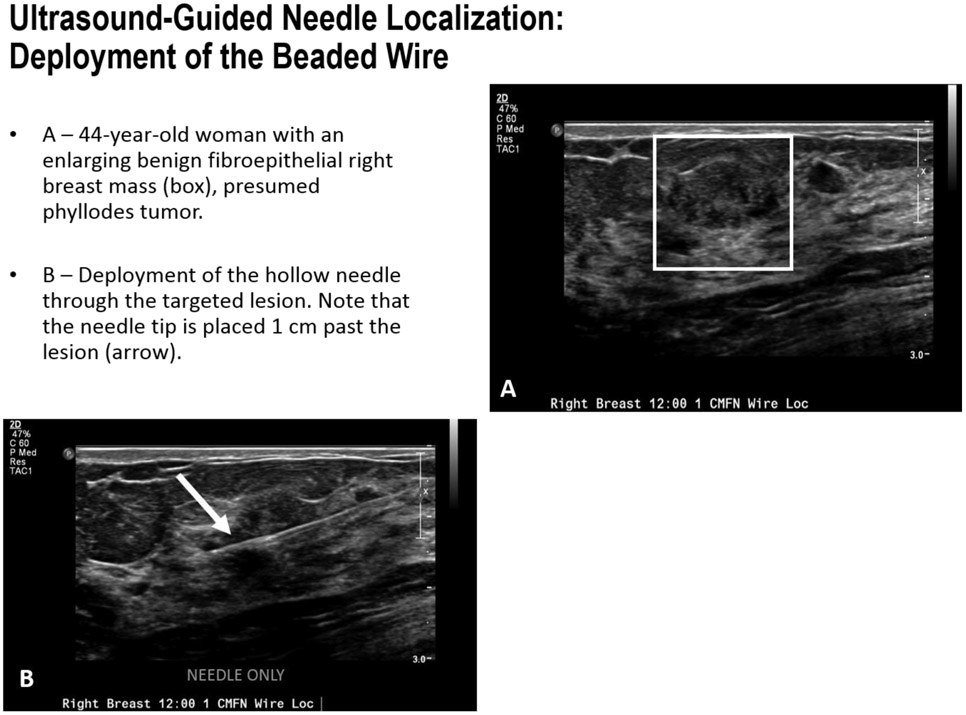
FIGURE 8A-B.
Ultrasound-Guided Needle Localization: Deployment of the Beaded Wire
FIGURE 8C-D.
Ultrasound-Guided Needle Localization: Deployment of the Beaded Wire
Acknowledgments
Disclosures: Supported by the NIH/NCI, Award # P30 CA016672
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Megan Kalambo, The University of Texas MD Anderson Cancer Center, Department of Radiology, 1155 Pressler, Unit 1350, CPB 5.3208, Houston, TX 77030, USA.
Basak E. Dogan, University of Texas Southwestern Medical Center, Department of Radiology, Breast Imaging Section, 2201 Inwood Road, Dallas, Texas 75390, USA.
Gary J. Whitman, The University of Texas MD Anderson Cancer Center, Department of Radiology, 1155 Pressler, Unit 1350, CPB 5.3208, Houston, TX 77030, USA.
References
- 1.Powell JL, Hawley JR, Lipari AM, et al. Impact of the addition of digital breast tomosynthesis (DBT) to standard 2D digital screening mammography on the rates of patient recall, cancer detection, and recommendations for short-term follow-up. Acad Radiol. 2017. March; 24(3):302–307. [DOI] [PubMed] [Google Scholar]
- 2.Cady B, Stone MD, Schuler JG, et al. The new era in breast cancer. Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch. Surg 1996;131:301–8. [DOI] [PubMed] [Google Scholar]
- 3.Agarwal S, Pappas L, Neumayer L, et al. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014;149:267–74. [DOI] [PubMed] [Google Scholar]
- 4.Mayo RC, Kalambo MJ, Parikh JR. Preoperative localization of breast lesions: Current techniques. Clin Imaging. 2019. Jul-Aug;56:1–8. [DOI] [PubMed] [Google Scholar]
- 5.Adrada BE, Huo L, Lane DL, Arribas EM, et al. Histopathologic correlation of residual mammographic microcalcifications after neoadjuvant chemotherapy for locally advanced breast cancer. Ann Surg Oncol. 2015. April; 22 (4): 111–7. [DOI] [PubMed] [Google Scholar]
- 6.Lee-Felker SA, Tekchandani L, Thomas M, et al. Newly Diagnosed Breast Cancer: Comparison of Contrast-enhanced Spectral Mammography and Breast MR Imaging in the Evaluation of Extent of Disease. Radiology. 2017. November;285(2):389–400. [DOI] [PubMed] [Google Scholar]
- 7.Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tevis SW, Neuman HB, Mittendorf EA, et al. Multidisciplinary intraoperative assessment of breast specimens reduces number of positive margins. Ann Surgical Oncol. October; 25(10):2932–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]