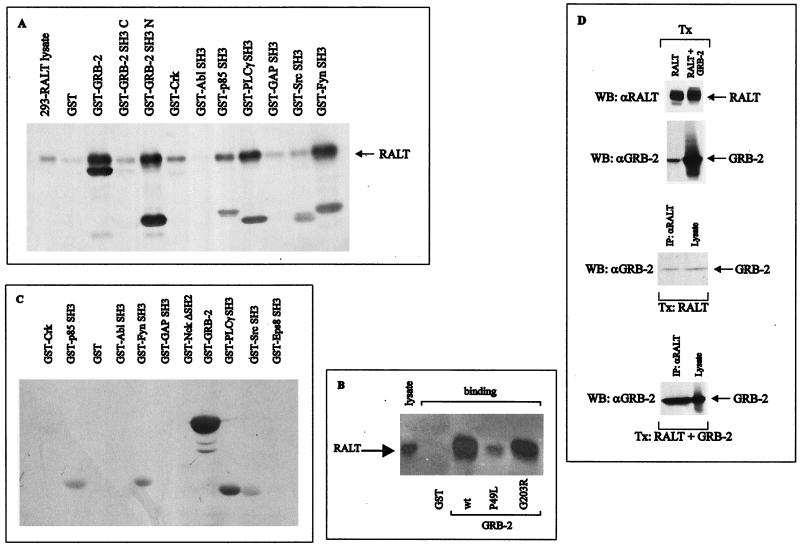
FIG. 6.
RALT binds to SH3 domains. (A) Lysates of 293-RALT transfectants were incubated with the indicated recombinant GST fusion proteins immobilized onto glutathione-agarose beads. Proteins bound to resins were analyzed by immunoblotting with anti-RALT antibodies and the ECL reaction. Lysate in the left lane represents 5% of the input lysate in the binding reaction. (B) GST–GRB-2 fusions expressed from either wt GRB-2 cDNA or cDNA encoding the indicated GRB-2 mutant proteins were purified onto glutathione-agarose beads in similar amounts and used as affinity reagents for the capture of RALT solubilized from 293 transfectants; lysate in the left lane corresponds to 5% input in the binding reaction. Proteins bound to resin were subjected to immunoblot analysis with S1 anti-RALT antibodies. (C) Each of the indicated recombinant proteins (2 μg; purified by affinity chromatography on glutathione-agarose resin) was run on SDS-PAGE gel and transferred to nitrocellulose filters. After the proteins were stained with Ponceau's red to ensure that equal amounts of protein were present in each lane, independent filters were probed with biotin-labeled GST–RALT 1-262 or GST–RALT 263-459; detection was with HRP-conjugated streptavidin followed by the ECL reaction. No specific reactivity was observed with RALT 1-262, and therefore only the filter probed with GST–RALT 263-459 is shown. (D) Lysates from 293 cells transfected with RALT or RALT and GRB-2 expression vectors were analyzed for RALT and GRB-2 expression by immunoblotting (upper two panels). Anti-RALT immunoprecipitates from these lysates were blotted with anti-GRB-2 antibodies along with lysates representing 10% of the input protein in the immunoprecipitation reaction (lower two panels). ECL exposure of the third panel from top was 90 s, while that of the bottom panel was 45 s. WB, Western blotting; Tx, transfection.