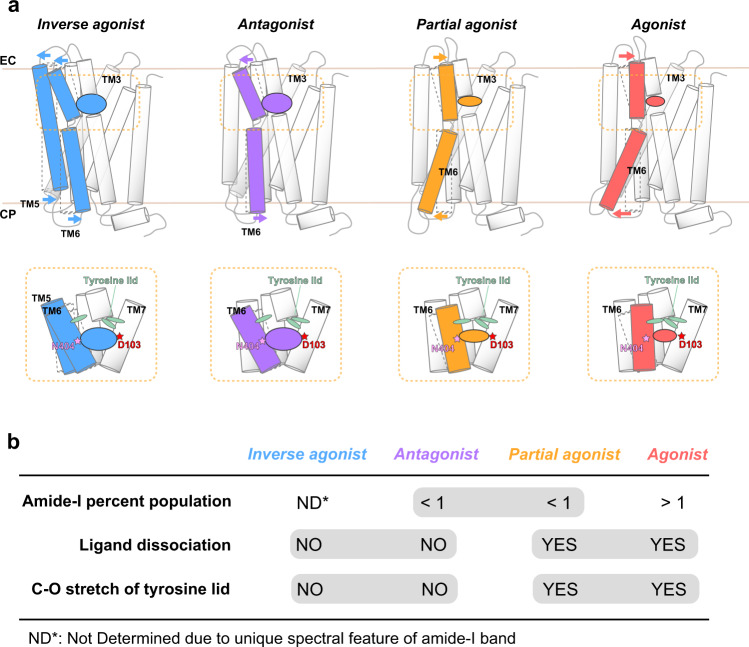
Fig. 5. Proposed conformational changes in M2R upon binding of ligands with different efficacies.
a (Upper) Schematic of M2R TM6 and TM5 conformational states upon binding of orthosteric ligands with different efficacies. TM6 features an open conformation in the extracellular side and a closed conformation in the cytoplasmic side upon binding with inverse agonist and antagonist. In contrast, TM6 features a closed conformation in the extracellular side and an open conformation in the cytoplasmic side upon binding with both agonist and partial agonist, which causes the opposite movement with both inverse agonist and antagonist. Yellow dotted line indicates the orthosteric ligand binding pocket. (Lower) Schematic of M2R ligand binding pocket surrounded by TM3, 5, 6, and 7. Two key amino acids (Asn4046.52 and Asp1033.32) and tyrosine lid are depicted by star and oval markers, respectively. Binding of either inverse agonist or antagonist opens the extracellular region of TM6, resulting in loosening of the tyrosine lid, whereas both agonist- and partial agonist-bound forms compacts the ligand-binding pocket, which induces the formation of tyrosine lid that excludes solvent entry. b Summary of the differences in the ATR-FTIR analysis of M2R with various ligand efficacies. By comprehensive comparing the three factors (amide-I band ratio, ligand dissociation, and C–O stretch of tyrosine lid) extracted from ATR- FTIR spectroscopy analysis, different ligand efficacy can be distinguished.