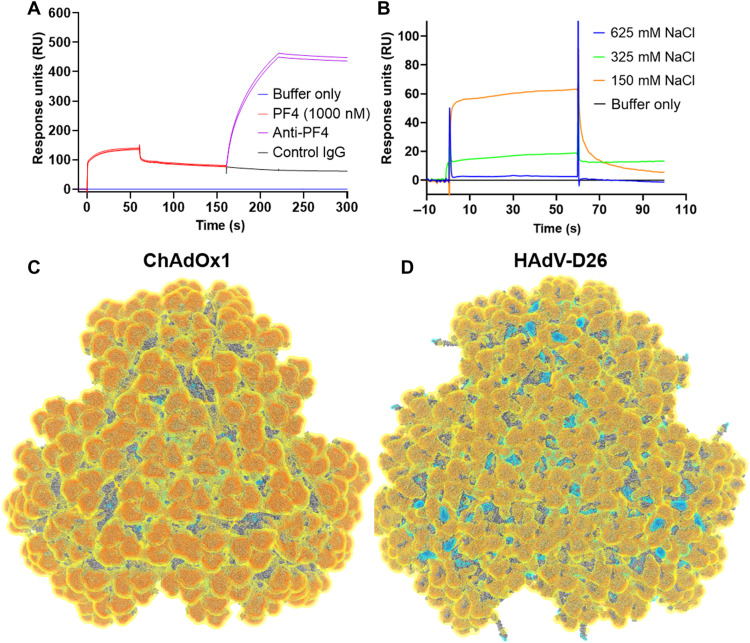
Fig. 5. ChAdOx1 creates a stable complex with PF4 and is highly electronegative.
A two-injection SPR experiment showed that, following PF4 binding to the ChAdOx1 capsid, a polyclonal αPF4 was able to bind to the surface of the chip indicating a ChAdOx1/PF4/antibody complex (A). IgG, immunoglobulin G. Similar SPR experiments show decreasing PF4 binding at increasing concentrations of NaCl (B). Visualization of the capsid (three vertices shown) of ChAdOx1 (C) and Ad26 (D) shows the electrostatic potential at −0.5 kBT (yellow), −1.0 kBT (orange), −1.5 kBT (red), 0.5 kBT (cyan), 1.0 kBT (blue), and 1.5 kBT (dark blue). Electronegative potential is focused at the apex of the hexons. ChAdOx1 is more electronegative than HAdV-D26 with negative charge extending into the interhexon spaces, while in HAdV-D26, it has electropositive charge in these regions. A more detailed capsid view is available in fig. S6.