FIGURE 1.
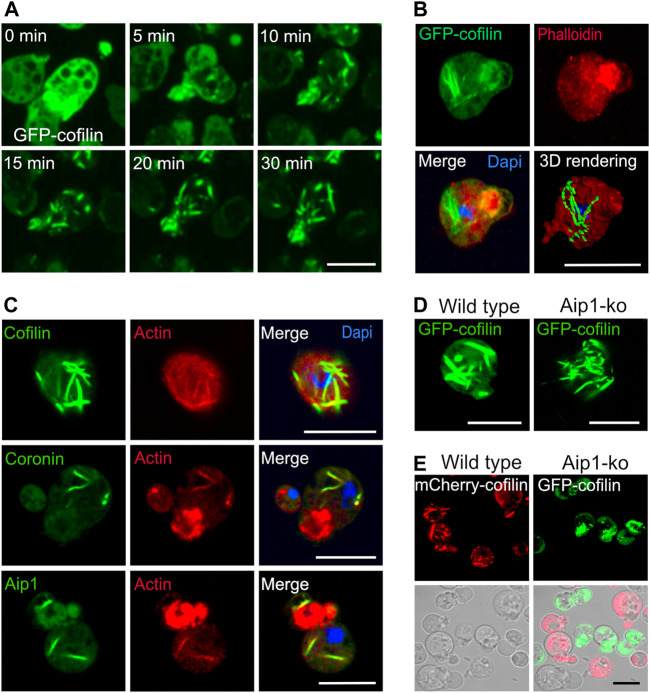
Cytoplasmic actin-cofilin rods induced by sodium azide. (A) Time series of confocal images of rod formation induced by 10 mM sodium azide. Dictyostelium cells expressing GFP-cofilin (green) were exposed to medium containing 10 mM sodium azide, and recorded by live-cell microscopy for 30 min. The time series show that already after 5 min of treatment, GFP-cofilin starts to redistribute from cortical areas into bundles of increasing size inside the cytoplasm. See also Supplementary Video S1 for the complete time series showing an overview of several cells. (B) Cytoplasmic actin-cofilin rods do not bind phalloidin. Dictyostelium cells expressing GFP-cofilin were fixed after 60 min of sodium azide treatment. GFP-cofilin (green) visualizes the cytoplasmic rods, phalloidin (red) is not co-localizing with GFP-cofilin rods. Dapi was used to label the nucleus. 3D rendering of the Z-stack projection was reconstructed using Imaris software. (C) Immunofluorescence labeling of cytoplasmic rods for the main constituents, actin and cofilin, as well as Aip1 and coronin after induction by sodium azide treatment in Dictyostelium wild-type cells. Cofilin, Aip1 and coronin were immunolabeled with the respective primary antibodies (green) and co-immunolabeled by an anti-actin antibody (red). Dapi was used to mark the nucleus (blue). Isotype controls are shown in Supplementary Figure S1A. (D) Rod formation after sodium azide treatment in Dictyostelium wild-type and Aip1-knockout (ko) cells expressing GFP-cofilin. (E) Dictyostelium cells expressing mCherry-cofilin and GFP-cofilin expressed in wild-type (red) or in Aip1-ko (green) cells were platted together and treated with sodium azide for 60 min. In the absence of Aip1, GFP-cofilin rods are not compacted into thicker bundles and remain more dispersed in comparison to wild-type cells. All scale bars, 10 µm.