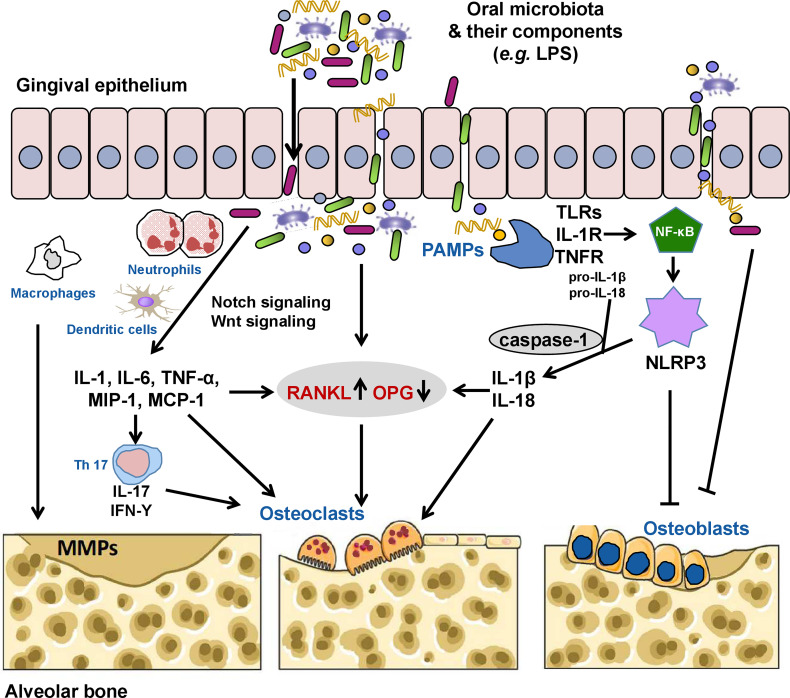
Figure 1.
The oral microbiota and its components can invade the gingival epithelium through the production of proteases, thus activating receptor activator of nuclear factor kappa B (NF-κB) ligand (RANKL) signaling directly or indirectly by inducing the secretion of inflammatory cytokines (interleukin [IL]-1, IL-6, tumor necrosis factor [TNF]-α, macrophage inflammatory protein [MIP]-1, and monocyte chemoattractant protein [MCP-1]) by neutrophils, macrophages, and dendritic cells, increasing the RANKL/osteoprotegerin (OPG) ratio and contributing to alveolar bone loss by inducing osteoclast formation. Pathogenic TH17 cells stimulated by bacterial invasion evokes periodontal immune responses against these microorganisms or their metabolites while also inducing bone damage. Some pathogens (e.g., Porphyromonas gingivalis) and their lipopolysaccharides (LPSs) can also directly induce the activation of matrix metalloproteinases (MMPs), which mediate the degradation of the extracellular matrix. Oral pathogen-associated molecular patterns (PAMPs) such as LPS, lipoteichoic acid, and double-stranded RNA can activate the innate immune system through pattern recognition receptors, including toll-like receptors (TLRs), IL-1 receptor (IL-1R), and TNF receptor (TNFR), causing the release of NF-κB into the nucleus to initiate the expression of the nucleotide oligomerization domain-like receptor family pyrin domain-containing 3 (NLRP3) inflammasome. Activated NLRP3 cleaves pro-caspase-1 into caspase-1. Caspase-1 promotes the maturation and release of pro-IL-1β and pro-IL-18 to induce secretion of RANKL and activate osteoclasts. NLRP3 and activated caspase-1 can also promote osteoblast apoptosis. In addition, the oral microbiota and/or microbial virulence factors can inhibit the differentiation and proliferation while promoting the apoptosis of osteoblasts via various mechanisms.