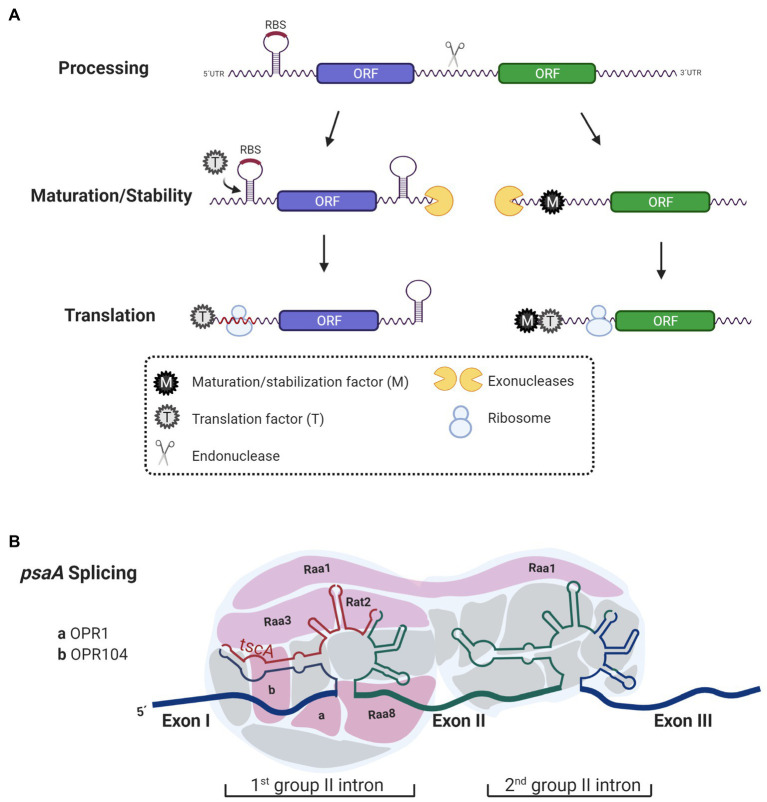
Figure 1.
Functions of Octo-, Tetra-, and Pentra-tricopeptide repeat (PPR) proteins. (A) Octo-tricopeptide repeat (OPR), tetra-tricopeptide repeat (TPR)-half-a-tetratricopeptide repeat (HAT), and PPR proteins act as maturation/stabilization (M) and translation factors (T). Before being translated, polycistronic transcripts are processed into monocistronic units by an endonuclease. The 5'- and 3'-ends generated after the cleavage are exposed and prone to degradation by exonucleases that trim RNA-ends until they are blocked by secondary structures in the mRNA or by RNA binding proteins, like OPR, HAT, and PPR proteins acting as M factors. Additionally, some transcripts need T factors (T) to promote and enhance translation. Some T factors anchor to mRNA and unmask ribosome binding sites (RBS) thus changing the secondary structure in the mRNA to facilitate ribosome biding to the mRNA. Some other T factors bind further away from the RBS and their mode of action activating translation is still unknown. (B) Some OPR proteins have been proposed to act as interaction modules for RNA processing. Some of the nucleus-encoded factors [RAA1, RAA3, RAT2, RAA8, OPR1 (Cre01.g001501), and OPR104 (Cre17.g698750)] that participate in the splicing of psaA that contain OPR motifs are shown. These OPR proteins interact with psaA mRNA facilitating splicing.