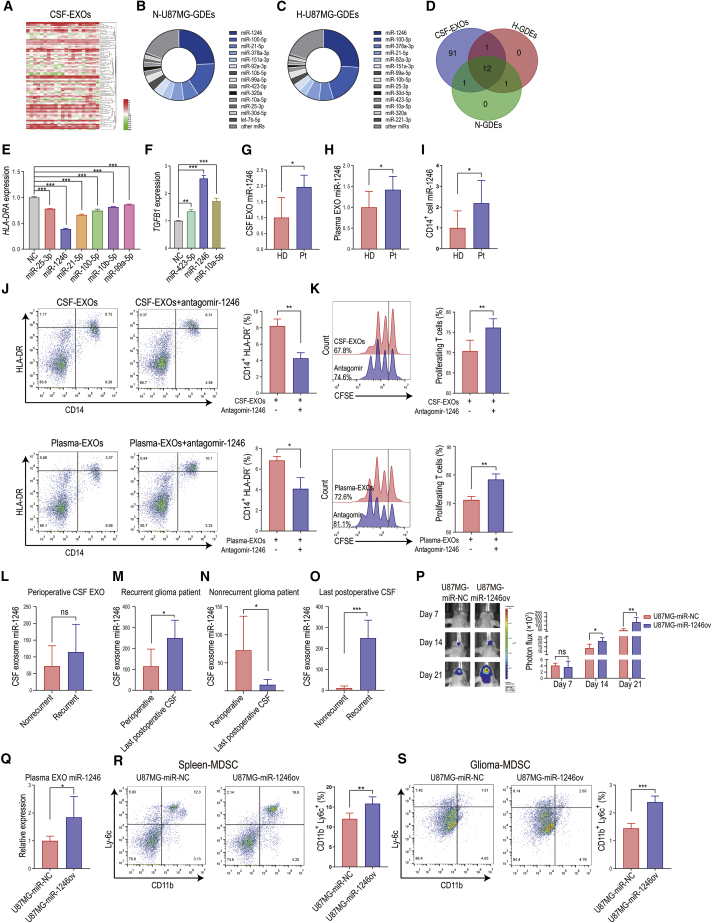
Figure 2.
Glioma patient CSF- and tumor cell-derived exosome miRNA sequencing identified miR-1246 as the key miRNA mediating the functional expansion of M-MDSCs
(A) Heatmap of differential CSF-exosome miRNA expression in 21 patients with gliomas of different grades. (B and C) Distribution of the top 14 enriched glioma exosomal miRNAs (higher than 1% of total miRNA) in normoxia- and hypoxia-conditioned glioma-derived exosomes (N-GDEs and H-GDEs, respectively). (D) Twelve miRNAs were shared among the miRNAs in all glioma patient CSF EXOs, N-GDEs, and H-GDEs. (E) Among the 12 miRNAs, miR-1246, miR-25-3p, miR-21-5p, miR-100-5p, miR-10b-5p, and miR-99a-5p overexpression in CD14+ cells reduced HLA-DRA expression. miR-1246 overexpression exhibited the strongest MDSC induction potency in CD14+ cells. (F) miR-1246, miR-423-5p, and miR-10a-5p increased gene expression of the immunosuppressive cytokine TGFB1. miR-1246 increased TGFB1 levels to the highest among all miRNAs tested. (G and H) The expression of miR-1246 was 1.96- and 1.42-fold higher in the EXOs of CSF (n = 5) and plasma (n = 7) collected from glioma patients than in those collected from healthy people. (I) miR-1246 expression increased 2.19-fold in CD14+ cells isolated from glioma patients (n = 7) compared to those isolated from healthy donors (n = 7). (J) Antagomir-1246 treatment impaired glioma patient CSF and plasma EXO-induced MDSC differentiation (n = 4 for each group, paired two-tailed Student’s t tests). (K) M-MDSCs obtained by stimulating CD14+ cells with glioma patient CSF EXOs or plasma EXOs were transfected with antagomir-1246 during EXOs stimulation. CFSE-labeled CD14-depleted PBMCs were co-cultured with M-MDSCs for 72 h. After 72 h of co-culture, CD8+ T cells among the above CD14-depleted PBMCs were labeled with anti-CD8 antibody and gated through flow cytometry. CD8+ T cell proliferation was evaluated by measuring the CFSE dilution signal (n = 4 for each group, paired two-tailed Student’s t tests). (L) No difference in EXO miR-1246 expression was identified in perioperative CSF collected from recurrent (n = 6) and nonrecurrent (n = 5) glioma patients. (M) In recurrent glioma patients, miR-1246 expression was significantly higher in the last postoperative CSF EXO sample than in the perioperative CSF EXO sample (n = 6). (N) In nonrecurrent glioma patients, EXO miR-1246 expression in the last postoperative CSF sample was reduced by 84.9% compared to that in perioperative CSF sample (n = 5). (O) miR-1246 expression in the last postoperative CSF sample from recurrent glioma patients (n = 6) was 22.9-fold higher than that in the postoperative CSF sample from nonrecurrent glioma patients (n = 5). (P) Bioluminescence images and luminescence quantification in mice implanted with control U87MG cells (U87MG-miR-NC) and miR-1246-overexpressing U87MG cells (U87MG-miR-1246ov) (n = 5 for each group). (Q) Plasma EXO miR-1246 expression was measured in glioma-bearing mice with quantitative real-time PCR (n = 5 for each group). (R and S) The percentage of CD11b+Ly6c+ M-MDSCs in spleens and tumors (n = 5 for each group). The data are presented as the mean ± SD; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗ p < 0.001.