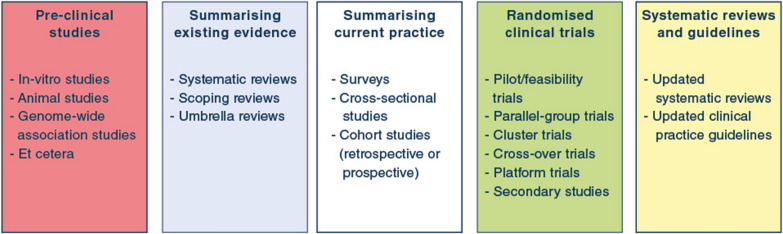
Fig. 2.
Overview of different study types and their role in clinical research programmes. In general, pre-clinical studies can provide necessary background or laboratory knowledge that may be used to generate hypotheses later assessed in clinical trials. Summarising existing evidence prior to start of clinical studies is sensible, to identify knowledge gaps, avoid duplication of efforts, and inform further clinical studies. Surveys may identify existing beliefs, practices and attitudes towards further studies; cross-sectional studies and cohort studies can describe prevalence, outcomes, predictors/risk factors and current practice. Randomised clinical trials remain the gold standard for intervention comparisons but may also provide data for secondary studies not necessarily focussing on the randomised intervention comparison. Before randomised clinical trials aimed at assessing efficacy or effectiveness of an intervention are conducted, pilot/feasibility trials may be conducted to prepare larger trials and assess protocol delivery and feasibility. Following the conduct of a randomised clinical trial, relevant systematic reviews and clinical practice guidelines should be updated as necessary, to ease implementation of trial results into clinical practice. Of note, the process is not always linear and unidirectional, and different study types may be conducted at different temporal stages during a research programme. Translational research may incorporate pre-clinical and laboratory studies and clinical studies, including non-randomised cohort studies and randomised clinical trials. Similarly, clinical studies may be used to collect data or samples that are further analysed outside the clinical setting