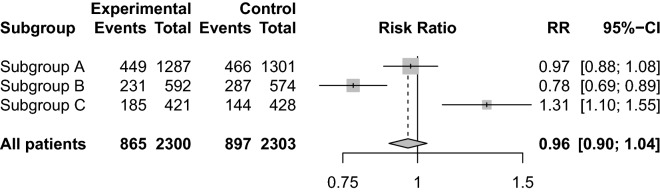
Fig. 4.
Heterogeneity of treatment effects in clinical trial. Forest plot illustrating a fictive clinical trial enrolling 4603 patients. In this trial, the average treatment effect may be considered neutral with a relative risk (RR) of 0.96 and 95% confidence interval of 0.90–1.04 (or inconclusive, if this interval included clinically relevant effects). The trial population consists of three fictive subgroups with heterogeneity of treatment effects: A, with an intervention effect that is neutral (or inconclusive), similarly to the pooled result; B, with substantial benefit from the intervention; and C, with substantial harm from the intervention. If only the average intervention effect is assessed, it may be concluded – based on the apparent neutral overall result – that whether the intervention or control is used has little influence on patient outcomes, and it may be missed that the intervention provides substantial benefit in some patients and substantial harm in others. Similarly, an intervention with an overall beneficial effect may be more beneficial in some subgroups than others and may provide harm in some patients, and vice versa