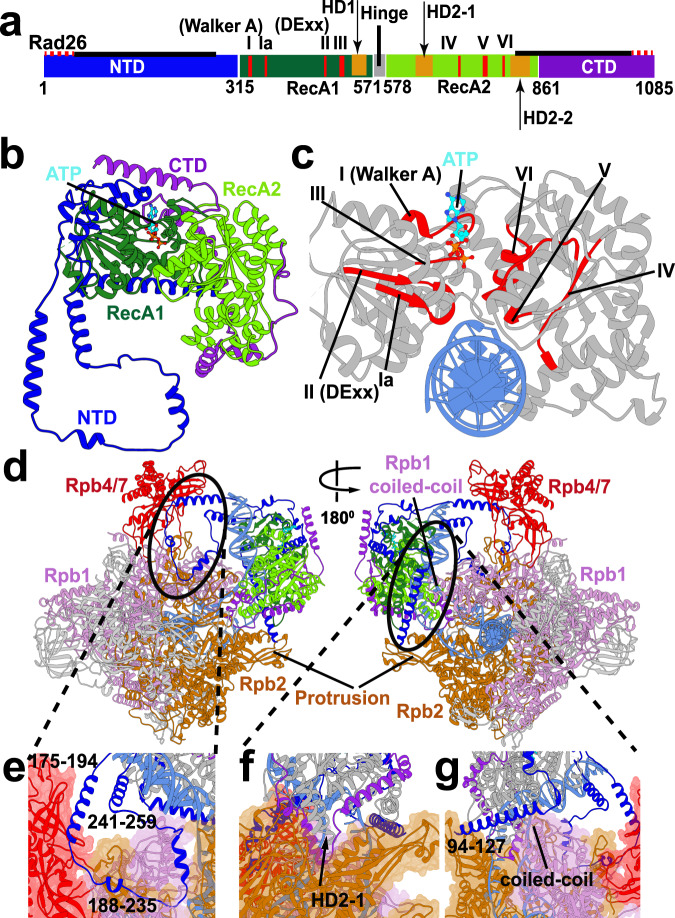
Fig. 1. Cryo-EM based Pol II–Rad26 structural model reveals the critical anchoring roles of the NTD and CTD domains.
a Schematic showing the Rad26 protein domains along its sequence. Newly modeled regions are highlighted with solid black lines. Seven canonical SF2 helicase motifs are shown in red bars. The hinge between the two ATPase modules is indicated by a black vertical bar. b View of Rad26 in cartoon representation colored by domains. RecA1 is shown in green, RecA2 in light green, NTD in blue and CTD in purple. c Side view of the Rad26 active site cleft with DNA (blue) and highlighted functional motifs I–VI (red). ATP is shown in stick representation and colored in cyan. d Anterior and posterior views of the Pol II–Rad26 complex. Rpb1, Rpb2 and Rpb4/7 are in light purple, brown and red, respectively. Rad26 is colored by domains. Circles demark zoomed-in regions in panels e-g. e NTD of Rad26 interacting with the Rpb1, Rpb2 and Rpb4/7 subunits of Pol II. f CTD and HD2-1 of Rad26 interacting with Rpb2 and DNA. g NTD of Rad26 interacting with the Rpb1 protrusion and the clamp coiled-coil domain.