Figure 4.
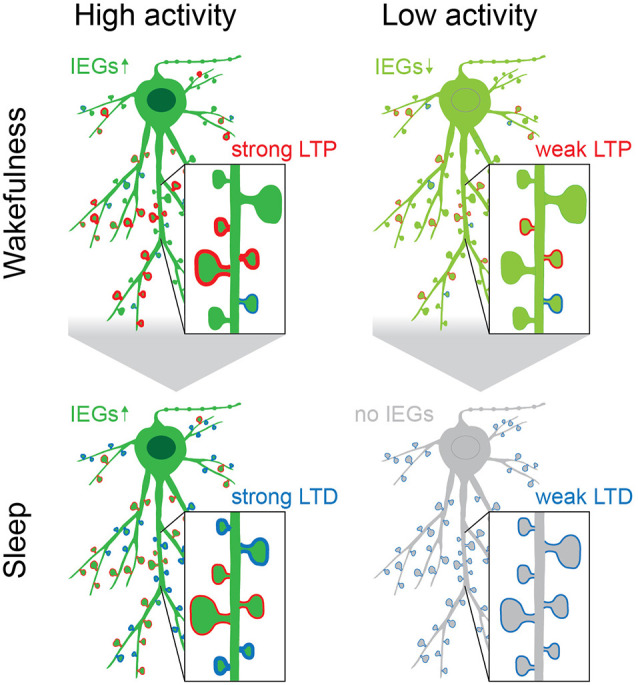
The acquisition of new memories during wakefulness causes upregulation of IEGs (green), which is followed by a second rise of IEG expression during sleep, preferentially in neurons that were highly active, exhibit increased intrinsic excitability and are constituents of initial memory engrams. The upregulation of IEGs during sleep is initiated during REM, following replay during NREM, and causes homeostatic plasticity (i.e., downscaling) to reduce noise and interferences from “old” or redundant memory traces (spines with blue rim). Spines that are reactivated during replay may be strengthened and protected from downscaling (spines with red rim; Rosanova and Ulrich, 2005; Chauvette et al., 2012; Kruskal et al., 2013; Yang et al., 2014; Atherton et al., 2015; Li et al., 2017; Lisman et al., 2018). This may happen in a spine- or dendrite-specific manner, as it has been shown that during sleep dendritic segments experience Ca2+-spikes that are separate from the cell body, as well as dendrite specific plasticity (Yang et al., 2014; Kastellakis et al., 2015; Li et al., 2017; Seibt et al., 2017). While the effects of IEGs during sleep may be most specific to neurons that are part of newly formed engrams (dark green neurons), the kinases GSK3β, Abl2, DCLK2, and CDK5 may act in concert to reduce synaptic strength on a global scale (Table 1, Diering et al., 2017; Bruning et al., 2019). Their combined action counterbalances the excess potentiation in the brain during wakefulness by “renormalizing” connections and thus aids the selective refinement of memory engrams (Cao et al., 2015; Attardo et al., 2018).
