Figure 6.
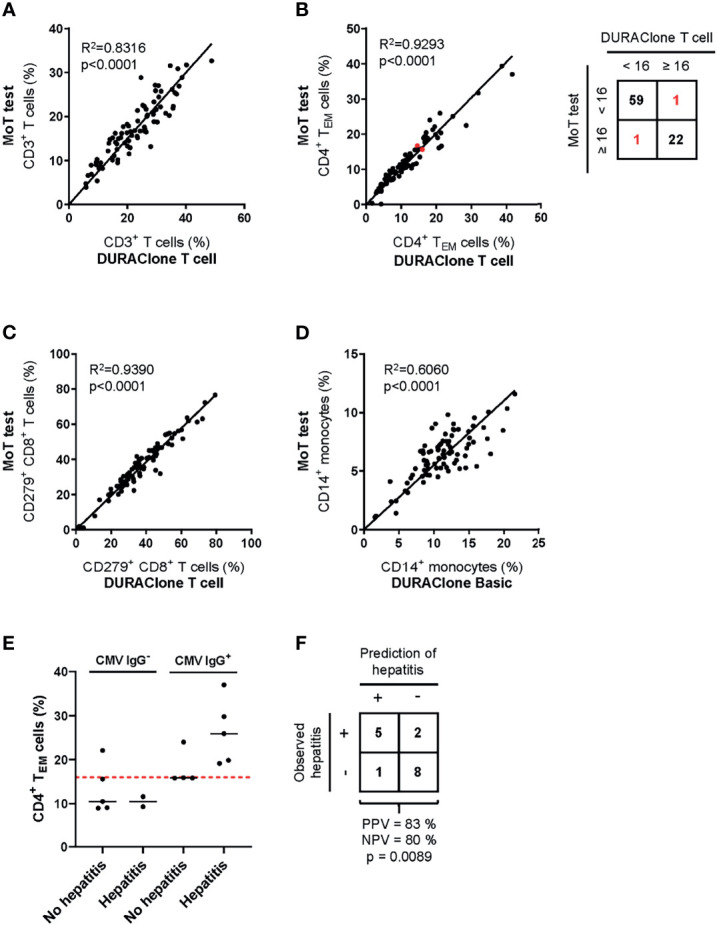
Method comparison of MoT test and DURAClone IM Tubes. Fresh whole blood EDTA samples from patients with stage II (n = 52), stage III (n = 11) or stage IV (n = 20) melanoma were investigated using the MoT test, the DURAClone IM Phenotyping Basic Tube and the DURAClone IM T cell subsets Tube. Frequencies of CD3+ T cells (A), CD4+ TEM cells (B), CD279+ CD8+ T cells (C) and CD14+ monocytes (D) were determined. The alternative methods differently classified only two samples with borderline CD4+ TEM cell frequencies (marked in red; method concordance of 98%). Fresh whole EDTA blood of stage IV melanoma patients (n = 16) was analyzed prior to checkpoint inhibitor treatment using the MoT test. (E) CMV serology status was determined and the development of immune-related hepatitis was evaluated within 12 weeks post therapy start. Dashed red line indicates a cut-off of CD4+ TEM ≥ 16%. Median values are indicated by a black line. (F) Classification of stage IV melanoma who did or did not develop immune-related hepatitis. Development of hepatitis was correctly predicted in 5 of 6 cases (PPV = 83%). 2 of 10 patients who developed hepatitis were incorrectly classified as hepatitis negative (NPV = 80%) (p = 0.0089, two-way ANOVA test).
