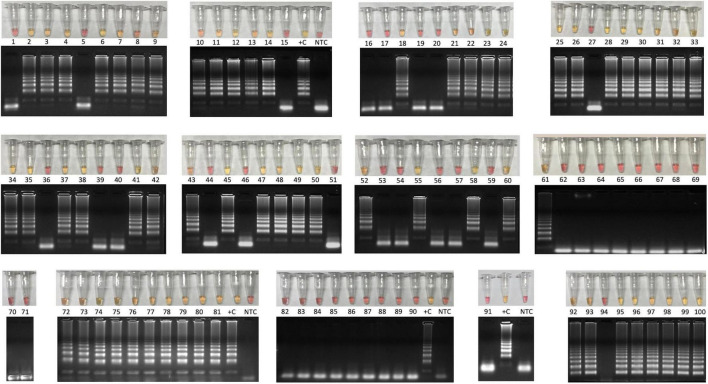
FIGURE 3.
Colorimetric RT-LAMP for COVID-19 diagnosis validation using 100 clinical samples. Clinical samples were collected from symptomatic and hospitalized patients by nasopharyngeal swabs in partnership with CT-Vacinas/UFMG, Belo Horizonte, Brazil. Samples were obtained from different parts including Brazilian Southeast and Northeast regions. The reaction was performed at 65°C during 30 min using WarmStart® colorimetric LAMP master mix (NEB #M1800) in 20 μL final volume. The RT-LAMP reaction targeted SARS-CoV-2 N gene. Yellow content indicates positive reaction, whereas the pink pattern reveals nonreagent samples. Amplicons were resolved in 2% agarose gel and stained with GelRed® (Biotium #41003) to confirm DNA amplification. Latter pattern confirmed specific SARS-CoV-2 amplification that matches with yellow output tubes, which is not observed in pink nonreagent tests. +C, positive control using RNA extracted from laboratory-Vero E6 cultured inactivated SARS-CoV-2; NTC, nontemplate control. Clinimetric parameters from these samples are presented in Supplementary Figure S1.