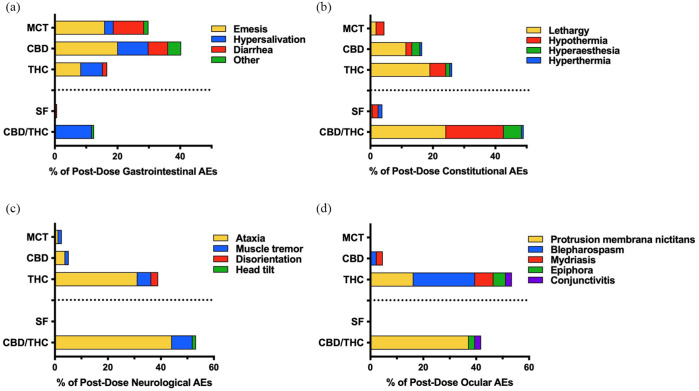
Figure 3.
Proportion and profile of post-dose adverse events (AEs) attributed to dose escalation of each oil in up to 11 doses (n = 4 per treatment). (a) Gastrointestinal, (b) constitutional, (c) neurologic and (d) ocular AEs were observed within 24 h of oil administration. ‘Other’ gastrointestinal AEs include abnormal excreta, bloody stool, retching and dehydration. MCT = medium-chain triglyceride oil; CBD = cannabidiol; THC = tetrahydrocannabinol; SF = sunflower oil