Abstract
Biodistribution studies of adenovirus-based vaccines support their clinical development by evaluating their spread and persistence following in vivo administration. AZD1222 (ChAdox1 nCov-19) is a replication-deficient non-human adenovirus-vectored vaccine for coronavirus disease 2019. In this nonclinical study, the biodistribution of AZD1222 was assessed in mice for 29 days following intramuscular injection. Results show that AZD1222 was safe and well tolerated, with a spread that was largely confined to administration sites and the proximal sciatic nerve, with low levels observed in sites that are involved in rapid clearance of particulates by the reticuloendothelial system. Accordingly, levels of AZD1222 decreased from Day 2 to Day 29, indicating clearance. There were no quantifiable levels of AZD1222 in the blood, brain, spinal cord, and reproductive tissue, suggesting a lack of widespread or long-term distribution of AZD1222 vector DNA throughout the body following its administration.
Keywords: Biodistribution, AZD1222, ChAdOx1 nCov-19, COVID-19
1. Introduction
AZD1222 (ChAdOx1 nCov-19) is a replication-deficient simian adenovirus-vectored vaccine for coronavirus disease 2019 (COVID-19) that encodes the full-length severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) spike protein [1]. Therapeutic DNA vaccines such as this can induce cellular and humoral immune responses to specific antigens, modulate the immune system, and are very stable, conferring several advantages over conventional vaccines [2], [3]. Viral vectors are the most frequently used carriers to deliver and protect DNA [2]. Furthermore, non-human adenoviral vectors avoid the risk of pre-existing neutralizing antibodies to the vaccine vector itself that are commonly observed with, and may limit the efficacy of, equivalent human adenoviral vaccine vectors [4].
Biodistribution analyses of new therapeutic DNA vaccines evaluate the spread and persistence of the vector to target and non-target tissues following direct administration in animals, and are routinely performed during product development. The biodistribution of DNA from different adenovector types has been evaluated in nonclinical studies, with results indicating that they are rapidly cleared from the body [5]. Furthermore, adenovectors have been shown to remain primarily at the site of administration in the muscle and subcutis, and variously trafficked to the liver, iliac lymph nodes, and spleen, but not to other distal organs, including remaining absent from the gonads. These seminal studies concluded that adenovirus vectors are safe and suitable for investigational human use, given intramuscularly, to prevent various infectious diseases [6].
Large phase 3 trials are designed to detect safety signals within prespecified timeframes, while pharmacovigilance of vaccine use in the real world may detect rare events that manifest only when tens of millions of people are vaccinated. Challenges lie in differentiating which safety signals from real-world use are related to the vaccine, and which have occurred coincidentally in the short risk interval after vaccine exposure. In very rare instances, a combination of thrombosis and thrombocytopenia has been reported following vaccination with COVID-19 vaccines, including AZD1222 [7], [8], [9], [10]. Independent safety reviews by regulatory authorities have, however, concluded that the balance of benefits and risks is firmly in favor of vaccination in adults of all age groups [11]. Biodistribution analyses may help evaluate the legitimacy of vaccine safety concerns by mapping the temporal dispersal of the vaccine following its administration.
Because AZD1222 is replication-incompetent in human cells and is administered via intramuscular injection, upon initial infection of cells, the biodistribution or further infection and spread of the adenovirus is expected to be negligible and clinically insignificant [12]. The objective of this study was, therefore, to determine the longitudinal and quantitative distribution of AZD1222 to multiple tissues (including the blood) following a single intramuscular injection in mice. Mice were chosen as they are considered immunologically relevant for vaccine toxicity testing, have previously been used to study HuCoV infection [13] and model COVID-19 [14], and AZD1222 induces robust antibody and cell-mediated immune responses in these animals [1].
2. Methods
Studies were conducted in an Organization for Economic Cooperation and Development (OECD) country and in accordance with OECD Test Guidelines and Principles of Good Laboratory Practice for nonclinical laboratory studies, complied with ARRIVE guidelines, and were conducted in accordance with the UK Animals (Scientific Procedures) Act, 1986.
The study protocol was developed in accordance with United States Food and Drug Administration, European Medicines Agency, Japanese, and World Health Organization guidelines for assessing the potential toxicity of vaccines for infectious diseases.
AZD1222 was the test agent. For control animals, AZD1222 vehicle was used.
A total of 160 (80 male, 80 female) CD-1 mice aged 6–8 weeks obtained from Charles River Laboratories (Charles River UK Limited, Margate, Kent, UK) were randomly allocated (1:1) to vaccine or control groups. For each animal, 0.035 mL of vaccine or control was administered intramuscularly in the thigh muscle of each hindlimb (0.07 mL in total). For the test group, the dose exceeded the human dose on a mg/kg basis, and was the highest feasible dose that can be administered to mice via intramuscular injection, considering the limitations of vaccine concentration and the total volume that can be administered given animal welfare considerations [15]. Animals were thoroughly examined before dosing and were observed regularly throughout the day of dosing and throughout the study period for potential vaccine-related reactions.
Following administration on Day 1, biodistribution of AZD1222 vector DNA (hereafter referred to as just AZD1222) to blood and feces was assessed on Days 2, 3, 5, and 9.
Biodistribution of AZD1222 to tissues (administration sites, adrenal gland, axillary lymph node, bone marrow, brain, heart, inguinal lymph node, kidney, liver, lung, mammary gland, mesenteric lymph node, ovaries, pancreas, sciatic nerve, spinal cord, spleen, testes, and thymus) was assessed on Days 2, 3, 9, or 29 following terminal anesthesia and complete necropsy examination.
Terminal anesthesia was performed on five male and five female animals from each study group at each timepoint. Separate instruments were used to extract different tissues. Bone marrow was collected from the left femur.
The concentration of AZD1222 relative to a DNA standard curve was determined by a validated method using quantitative polymerase chain reaction. Regression analysis was performed using Watson LIMS system (Version 7.4.2, Thermo-Fisher, Waltham, MA, USA). Sample results > limit of detection (LOD) (10 copies/reaction) were back-calculated to AZD1222 vector DNA concentration in copies/μg DNA. Samples between the LOD and lower limit of quantitation (LLOQ) of the assay (10 and 50 copies/reaction, respectively) were flagged in the corresponding tables as < LLOQ.
3. Results
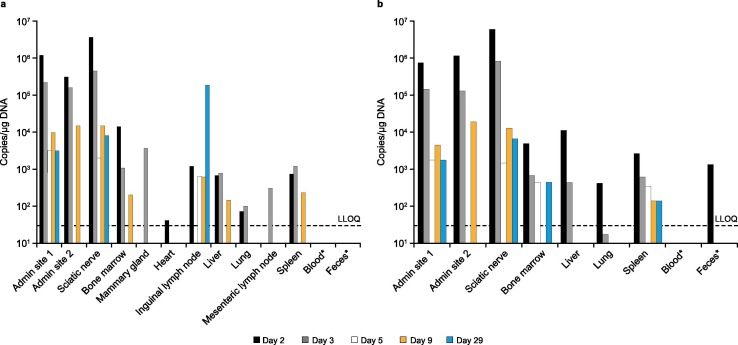
AZD1222 was well-tolerated, and throughout the study there were no clinical observations, unscheduled deaths, changes in bodyweight, or changes in food consumption that were considered related to the administration of the vaccine. Furthermore, there were no quantifiable concentrations of AZD1222 in blood or feces samples collected from control or dosed animals, except for one feces sample from a dosed animal at Day 2 (1.03 × 103 copies/μg DNA) Fig. 1 . In dosed animals, and across all timepoints, the highest levels of AZD1222 (>104 –107 copies/μg DNA) were observed in the two administration sites and the sciatic nerve (proximal to the injection site) with decreased levels for most animals at Day 9 and 29 (<104 copies/ μg DNA) Fig. 1. Lower levels of AZD1222 (<LLOQ − 104 copies/μg DNA) were observed in the bone marrow, liver, spleen, lymph nodes, and lungs of dosed animals Fig. 1. In all other tissues sampled, including the brain, spinal cord, and reproductive tissue, there were no quantifiable levels of AZD1222 in any animals at any timepoint except for one mammary gland sample from a dosed male at Day 3 (3.68 × 103 copies/μg DNA). The levels of AZD1222 and the number of tissues with detectable levels of AZD1222 decreased from Days 2 to 29 Fig. 1. Sentinel controls were negative for all batches, and all results from the reported data met the acceptance criteria set during the method validation. The reported results are, therefore, considered representative of the concentration of AZD1222 in blood, feces, and tissue samples for this study.
Fig. 1.
Biodistribution of AZD1222 following a single intramuscular injection in (a) male and (b) female mice. Day 29 analyses were not performed on blood and feces samples. LLOQ, lower limit of quantification.
4. Discussion
The biodistribution of AZD1222 was largely confined to administration sites and the sciatic nerve following intramuscular injection in mice, and, importantly, was not detected in the majority of tissues sampled, including brain, spinal cord, and reproductive tissue, and was also not detected in blood. The absence of detectable AZD1222 in brain and spinal cord suggests there is negligible risk of an autoimmune response targeting the nervous system, whilst the absence of detectable AZD1222 in reproductive tissues suggests negligible risk of vertical transmission of the vector. During the course of the study, from Day 2 to Day 29, the number of animals with positive tissues and the DNA copy numbers present in those tissues declined, indicating clearance. The low levels of AZD1222 detected at Day 29 could be because of low turnover of transduced cells.
The sciatic nerve lies in close proximity to the injection site, and consistent with findings from the repeat dose toxicity study, histopathological examination of the main study animals identified mononuclear and/or mixed cell inflammation in the subcutaneous tissue and underlying skeletal muscle at control and AZD1222 administration sites. This occurred at a higher incidence in animals dosed with AZD1222 compared with control animals. In some animals there was an extended distribution of inflammatory cells into the fascia and connective tissue below the skeletal muscle at the administration sites, which extended to surround the sciatic nerve. The inflammatory cell distribution did not extend into the endoneurium of the sciatic nerve and no findings were present in the underlying axons, which appeared histologically normal. Inflammatory cells were not observed in the nerve roots contained within the lumbar spinal cord sections, confirming that the epineural/perineural inflammatory cells noted in the sciatic nerve samples resulted from an extension of the inflammation from the adjacent injection site. There were no findings in the administration sites or sciatic nerves at the end of the recovery period, indicating complete recovery of the AZD1222-related inflammation [16].
Results from this study show that AZD1222 was not detected in the blood of dosed animals. The mechanism for very rare events of thrombosis with thrombocytopenia following vaccination with AZD1222 is still under investigation but has been theorized to involve the induction of anti-platelet factor 4 (PF4) antibodies, which are thought to mimic the effect of heparin by binding to a similar site on PF4. PF4 tetramers then purportedly cluster to form immune complexes which, via the engagement of FcγRIIa receptors, activate platelets and initiate coagulation to cause thrombocytopenia and thrombosis [9], [10], [17]. Some studies on the other hand have shown that thrombotic events can be triggered by SARS-CoV-2 itself [18] at a far higher frequency than occurs following COVID-19 vaccine exposure, and with an increased risk of death [19].
The biodistribution and persistence of DNA vaccines are influenced by the type of expression vector used, as well as the route of administration [2]. On the basis of platform data, it was predicted that intramuscular injection of this adenovirus-vectored vaccine AZD1222 would not have clinically significant biodistribution [12], which was duly shown in these studies. The absence of significant levels of viral vector in the blood, but low levels detected in tissues distal to the site of injection (ie, liver, spleen, lung, and bone marrow) is due to the short half-life of viral particles in the blood, and the accumulation of viral vector or fragments thereof at sites that are involved in rapid clearance of particulates by the reticuloendothelial system in the liver, spleen and bone marrow [5].
In conclusion, results from this study show that AZD1222 delivered intramuscularly to mice was safe and did not result in either long-term or widespread distribution of vector DNA throughout the body.
Author Contributions
All authors contributed to the study design, and to the collection, analysis and interpretation of data. They also contributed to the development of the manuscript, including review of drafts, and approval of the final draft for submission. All authors agree to be personally accountable for their own contributions and for ensuring that questions relating to the accuracy or integrity of any part of the work, even ones in which the author was not personally involved, are appropriately investigated, resolved, and documented in the literature.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: [All authors are employed by AstraZeneca].
Acknowledgments
Acknowledgements
Medical writing and editing assistance, including revision of drafts under the direction and guidance of the authors, incorporating author feedback, and manuscript submission, was provided by Lucy Bee, Payal Jain and Heather Shawcross (Fishawack Health), in accordance with Good Publication Practice (GPP3) guidelines (Ann Intern Med. 2015;163:461–4). This support was funded by AstraZeneca. The study was conducted at Charles River Laboratories, Edinburgh, and animals were provided by Charles River (Charles River UK Limited, Margate, Kent, UK). The authors thank Professor Dame Sarah Gilbert, FMedSci at the University of Oxford for advice and for help with the supply of test material for preliminary studies.
Funding
This study was supported by AstraZeneca.
Data Sharing
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure
References
- 1.Graham S.P., McLean R.K., Spencer A.J., Belij-Rammerstorfer S., Wright D., Ulaszewska M., et al. Evaluation of the immunogenicity of prime-boost vaccination with the replication-deficient viral vectored COVID-19 vaccine candidate ChAdOx1 nCoV-19. npj Vaccines. 2020;5(1) doi: 10.1038/s41541-020-00221-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhao X., Long J., Liang F., Liu N., Sun Y., Xi Y. Dynamic profiles, biodistribution and integration evaluation after intramuscular/intravenous delivery of a novel therapeutic DNA vaccine encoding chicken type II collagen for rheumatoid arthritis in vaccinated normal rodent. J Nanobiotechnol. 2019;17(1):94. doi: 10.1186/s12951-019-0528-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schalk J.A.C., Mooi F.R., Berbers G.A.M., van Aerts L.A., Ovelgönne H., Kimman T.G. Preclinical and clinical safety studies on DNA vaccines. Hum Vaccin. 2006;2(2):45–53. doi: 10.4161/hv.2.2.2620. [DOI] [PubMed] [Google Scholar]
- 4.Tatsis N., Ertl H.C.J. Adenoviruses as vaccine vectors. Mol Ther. 2004;10(4):616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alemany R., Suzuki K., Curiel D.T. Blood clearance rates of adenovirus type 5 in mice. J Gen Virol. 2000;81(Pt 11):2605–2609. doi: 10.1099/0022-1317-81-11-2605. [DOI] [PubMed] [Google Scholar]
- 6.Sheets R.L., Stein J., Bailer R.T., Koup R.A., Andrews C., Nason M., et al. Biodistribution and toxicological safety of adenovirus type 5 and type 35 vectored vaccines against human immunodeficiency virus-1 (HIV-1), Ebola, or Marburg are similar despite differing adenovirus serotype vector, manufacturer's construct, or gene inserts. J Immunotoxicol. 2008;5(3):315–335. doi: 10.1080/15376510802312464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muir K.-L., Kallam A., Koepsell S.A., Gundabolu K. Thrombotic Thrombocytopenia after Ad26.COV2.S Vaccination. N Engl J Med. 2021;384(20):1964–1965. doi: 10.1056/NEJMc2105869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cines D.B., Bussel J.B. SARS-CoV-2 Vaccine-Induced Immune Thrombotic Thrombocytopenia. N Engl J Med. 2021;384(23):2254–2256. doi: 10.1056/NEJMe2106315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greinacher A., Thiele T., Warkentin T.E., Weisser K., Kyrle P.A., Eichinger S. Thrombotic Thrombocytopenia after ChAdOx1 nCov-19 Vaccination. N Engl J Med. 2021;384(22):2092–2101. doi: 10.1056/NEJMoa2104840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schultz N.H., Sørvoll I.H., Michelsen A.E., Munthe L.A., Lund-Johansen F., Ahlen M.T., et al. Thrombosis and Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. N Engl J Med. 2021;384(22):2124–2130. doi: 10.1056/NEJMoa2104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.European Medicines Agency. AstraZeneca's COVID-19 vaccine: benefits and risk in context. 2021.
- 12.European Medicines Agency. Assessment Report: COVID-19 Vaccine AstraZeneca. 2021.
- 13.Jonsdottir H.R., Dijkman R. Coronaviruses and the human airway: a universal system for virus-host interaction studies. Virol J. 2016;13(1) doi: 10.1186/s12985-016-0479-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S., Yadav P.K., Srinivasan R., Perumal N. Selection of animal models for COVID-19 research. Virusdisease. 2020;31(4):453–458. doi: 10.1007/s13337-020-00637-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization. WHO Guidelines on Nonclinical Evaluation of Vaccines 2005 [Available from: https://www.who.int/biologicals/publications/trs/areas/vaccines/nonclinical_evaluation/ANNEX%201Nonclinical.P31-63.pdf?ua=1.
- 16.European Medicines Agency. COVID-19 Vaccine AstraZeneca 2021 [Available from: https://www.ema.europa.eu/en/documents/product-information/covid-19-vaccine-astrazeneca-product-information-approved-chmp-29-january-2021-pending-endorsement_en.pdf.
- 17.Huynh A., Kelton J.G., Arnold D.M., Daka M., Nazy I. Antibody epitopes in vaccine-induced immune thrombotic thrombocytopaenia. Nature. 2021;596(7873):565–569. doi: 10.1038/s41586-021-03744-4. [DOI] [PubMed] [Google Scholar]
- 18.Nishiga M., Wang D.W., Han Y., Lewis D.B., Wu J.C. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17(9):543–558. doi: 10.1038/s41569-020-0413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Malas M.B., Naazie I.N., Elsayed N., Mathlouthi A., Marmor R., Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: A systematic review and meta-analysis. EClinicalMedicine. 2020;29-30:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]