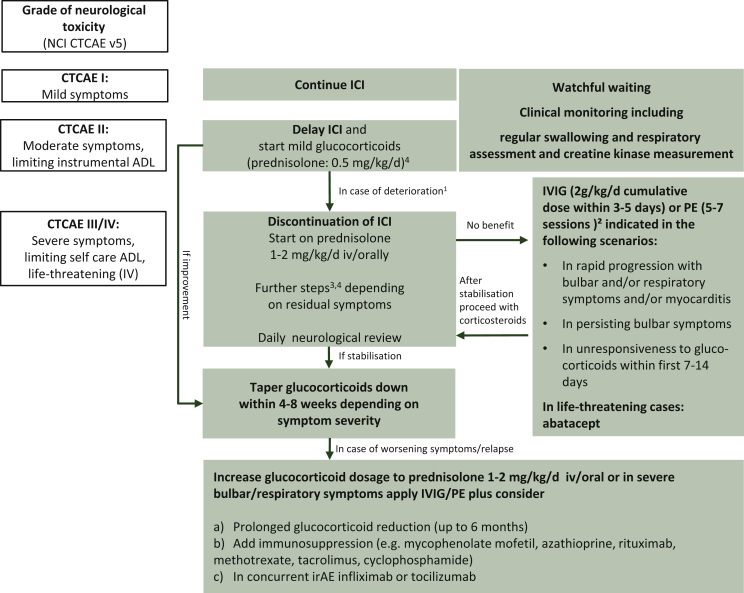
Figure 1.
Suggested therapeutic management algorithm according to severity of neuromuscular irAE, (all recommendations are based on level of evidence V in accordance with the ESMO guidelines2).
Re-challenge of ICI only if: symptoms remitted or at least stabilized, free or on low-level glucocorticoids for 4-8 weeks; convincing initial response to therapy, preceding CTCAE I/II irAE; follow more closely the more severe initial irAEs were.
ADL, activities of daily living; CTCAE, Common Terminology Criteria for Adverse Events; irAE, immune-related adverse event; ICI, immune checkpoint inhibitor; IVIG, intravenous immunoglobulins; NCI, National Cancer Institute; PE, plasma exchange.
1Timely consultation of a neurologist.
2In life-threatening symptoms, PE might be the favourable option; consider contraindications: renal failure, hypercoagulable states, sepsis, haemodynamic instability.
3Glucocorticoids are usually not recommended for idiopathic Guillain-Barré syndrome, however in mild ICI-related forms, a trial is reasonable (methylprednisolone 2-4 mg/kg/day), followed by slow glucocorticoid taper.
4Pulse corticosteroid dosing (methylprednisolone 1 g/day for 5 days) may also be considered for G3-4 along with IVIG or plasmapheresis.
5In all scenarios pyridostigmine starting from 3 × 30 mg orally up to 600 mg daily may be used in case of myasthenic symptoms. A dose of 30 mg oral pyridostigmine corresponds to 1 mg pyridostigmine intravenously or 0.75 mg neostigmine intramuscularly. In case of intubation pyridostigmine may be discontinued/on hold.