Fig. 1.
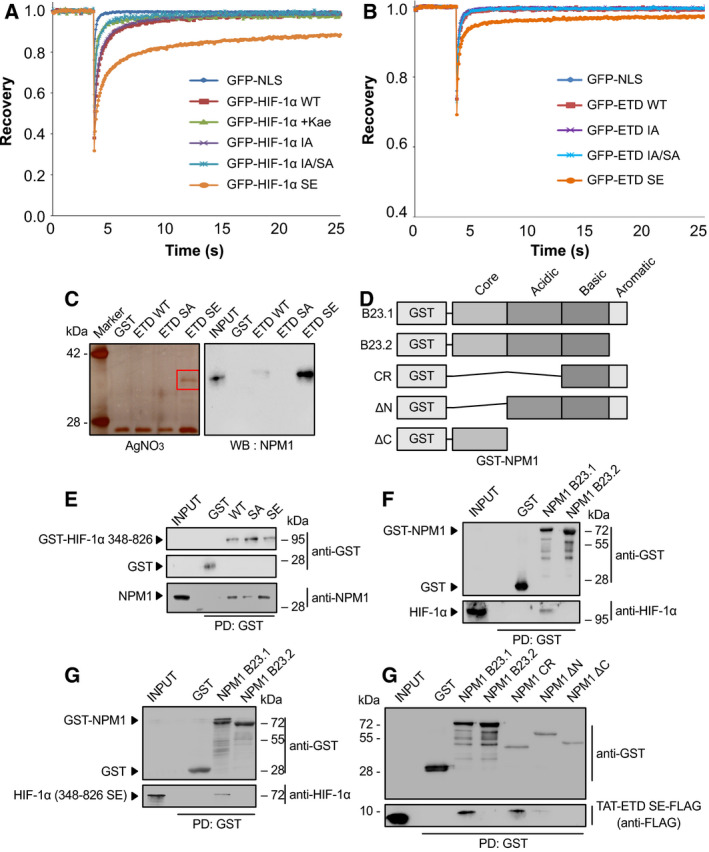
Identification of NPM1 as a phosphorylation‐dependent and direct interaction partner of HIF‐1α. (A,B) Phosphorylation of HIF‐1α by ERK increases its affinity for intranuclear immobile elements. Huh7 cells overexpressing the indicated full‐length GFP‐tagged (A) HIF‐1α or (B) HIF‐1α ETD forms were grown under normoxia and analyzed with FRAP 24 h post‐transfection. When needed, cells were treated with 50 μμ of kaempferol for 4 h as specified (WT+kae), in order to inhibit ERK activation. Curves represent the mean corrected fluorescence intensities over time. Curves represent the mean of two independent experiments (number of analyzed cells (n) are given in Table S4; ± SD for each curve is shown in Figs S2 and S3. (C) Analysis of HeLa cell proteins bound to different recombinant, purified, and immobilized on GSH‐agarose mutant forms of the GST‐HIF‐1α(ETD) (as indicated) after their elution by TEV‐mediated cleavage of the GST moiety. Left panel: Analysis by SDS/PAGE/AgNO3 staining (red square specifies gel section analyzed by mass spectrometry). Right panel: Analysis by western blotting (WB) with an anti‐NPM1 antibody. Images are representative of three independent experiments (see also Fig. S4A). (D) Schematic representation of domain structure of full‐length GST‐tagged NPM1 isoforms (B23.1 or B23.2) or their truncation forms CR, ΔN, and ΔC used in the following in vitro binding assays. (E) Soluble HeLa protein extracts (INPUT) were mixed with GSH‐agarose beads carrying GST or different mutant forms of GST–HIF‐1α(348–826) (as indicated), and bound proteins (PD: Pull‐Down) were analyzed by immunoblotting using antibodies against GST or NPM1 proteins. (F–H) GSH‐agarose beads carrying GST alone or different bacterially expressed and purified forms/domains of GST‐NPM1 were mixed with soluble protein extracts from hypoxic HeLa cells (F; INPUT) or bacterially expressed and purified phosphomimetic mutant HIF‐1α(348–826)SE (G; INPUT) or bacterially expressed and purified phosphomimetic TAT‐ETD‐SE‐FLAG peptide (H; INPUT) and bound proteins (PD: Pull‐Down) were analyzed by immunoblotting using antibodies against GST, HIF‐1α, and Flag (as indicated). Panels in C, E, F, G, H show single blot areas that correspond to the indicated molecular weight marker and were cut after blotting for analysis with different antibodies; images in E, F, G, H are representative of two independent experiments.
