Figure 5.
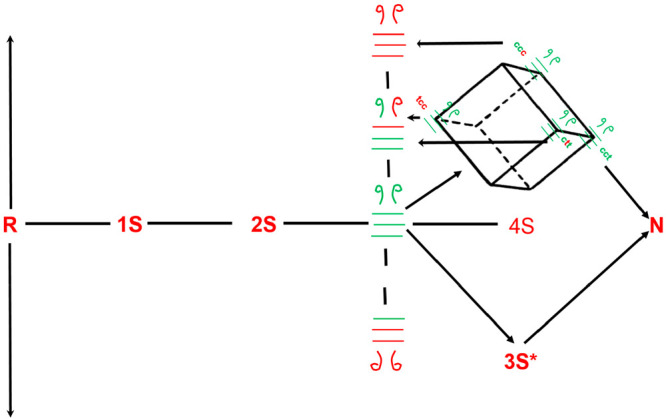
Cis–trans proline isomerization around the X–Pro peptide bond competes with the reshuffling reactions. Only native disulfide-bond-containing 3S species with their X–Pro peptide bonds in the c,c,t conformation can conformationally fold to form the 3S* species. The remaining X–pro isomers (e.g., c,t,c) must isomerize to c,c,t prior to conformational folding. However, since proline isomerization is slow, there is a high likelyhood that the native disulfide-bond-containing 3S intermediate (all green bonds with c,t,c disposition) reshuffles to a non-native disulfide-bond-containing isomer.
