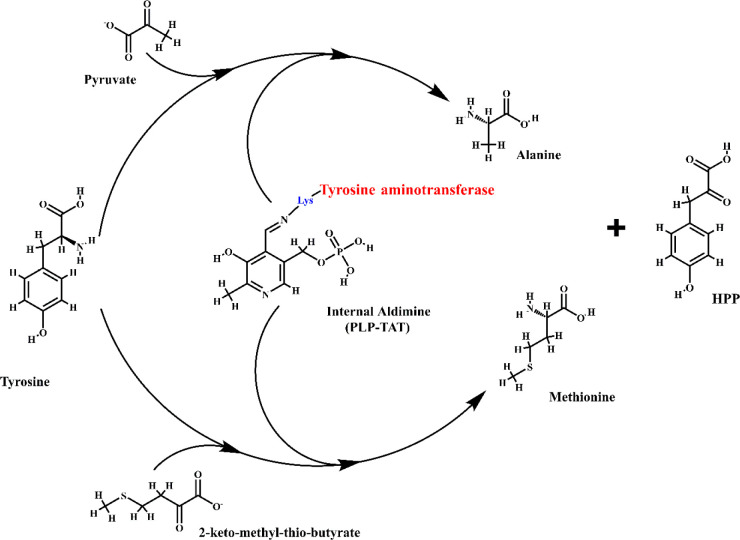
Figure 1.
Tyrosine aminotransferase (TAT) enzyme mechanism. Tyrosine aminotransferase is unique in trypanosomatids as they utilize pyruvate as a cosubstrate rather than α-ketoglutarate as observed in mammals. The transamination mechanism proceeds in two steps wherein tyrosine act as the amino donor and pyruvate or 2-ketomethylthiobutyrate as the amino acceptor. An internal aldimine (PLP-TAT) is formed between the active site lysine (in blue) of TAT (in red) and the carbonyl group of pyridoxal-l-phosphate (PLP). The internal aldimine then facilitates the transfer of the amino group. The end products formed from pyruvate/2-ketomethylthiobutyrate are alanine/methionine, respectively, along with 4-hydroxyphenylpyruvate (HPP) formed from tyrosine, which is utilized in the next step of the tocopherol pathway.