Figure 2.
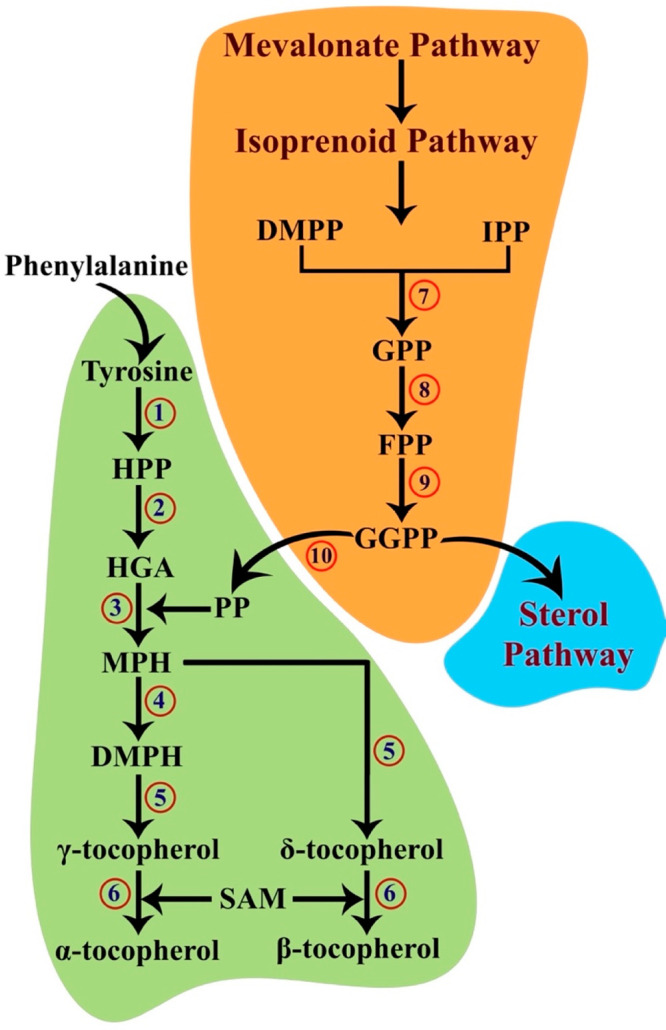
Hypothesized tocopherol biosynthesis pathway. Tyrosine produced as a result of phenylalanine hydroxylase is the first substrate in the tocopherol pathway. The produced tyrosine immediately transfers an amino group to a ketoacid and transmutes into 4-hydroxyphenylpyruvate (HPP). HPP is converted in the presence of oxygen and Fe2+ into homogentisate (HGA). The consequent reaction requires phytyl phosphate (PP) produced by a parallel mevalonate–isoprenoid pathway. The downstream isoprenoid focuses on the production of sterols and contains isopentyl diphosphate (IPP), dimethylallyl diphosphate (DMPP), geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP). PP is produced when GGPP is reduced. The phytyl group of PP is transferred to HGA, giving rise to 2-methyl-6-phytylhydroquinone (MPH), and a methyl group is then transferred to MPH to result in 2,3-dimethyl-5-phytylhydroquinone (DMPH). DMPH and MPH are then subjected to cyclization to produce the γ- and δ-homologues of tocopherols. The α- and β-homologues are formed by transferring a methyl group from S-adenosyl-l-methionine to the formerly synthesized homologues: 1, tyrosine aminotransferase; 2, 4-hydroxyphenylpyruvate dioxygenase; 3, homogentisate phytyl transferase; 4, 2-methyl-6-phytylhydroquinone methyltransferase; 5, tocopherol cyclase; 6, γ-tocopherol methyltransferase; 7, geranyl/farnesyl diphosphate synthase; 8, geranyl/farnesyl diphosphate synthase; 9, geranylgeranyl diphosphate synthase; 10, geranylgeranyl diphosphate reductase.
