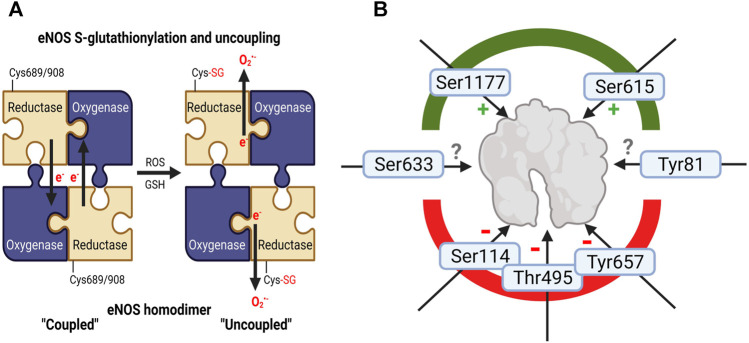
FIGURE 3.
Adverse regulation of eNOS function by noise. (A) Schematic explanation of increased eNOS S-glutathionylation in mouse tissues (a surrogate marker for uncoupling of the protein) upon noise exposure (Munzel et al., 2017; Kroller-Schon et al., 2018; Steven et al., 2020). In the “coupled” eNOS homodimer, electrons are usually transferred from the NADPH and flavins to the hem iron. Cysteine residues 689 and/or 908 undergo S-glutathionylation with structural changes (Chen et al., 2010), followed by misdirection of the electrons to molecular oxygen and superoxide formation, termed “uncoupled” state of eNOS. (B) eNOS activity is regulated by various kinase-dependent modifications such as activating phosphorylation at serine 1177 or Ser615 and inactivating ones at serine 114, threonine 495 (or 497 depending on the species) and tyrosine 657 (Fleming and Busse, 2003; Mount et al., 2007). Although pThr495-and pTyr657-eNOS was not reported for noise exposure, these inactivating phosphorylations may be expected since they are mediated by oxidatively activated kinases (PKC and PYK-2). Whereas higher eNOS protein expression and Ser1177 phosphorylation was observed in noise (4d)-exposed mice indicating counterregulatory upregulation and activating modification to rescue uncoupling of eNOS enzyme (Munzel et al., 2017; Kroller-Schon et al., 2018), suppression of pSer1177-eNOS was observed in noise-exposed hypertensive mice exposed to 7 days of noise (Steven et al., 2020). Other eNOS phosphorylation sites are not completely explored with respect to their functional effects (Ser633 and Tyr81). Image was created using Biorender.com.