Abstract
BACKGROUND
Previous studies had shown endoscopic retrograde appendicitis therapy (ERAT) is an effective treatment for acute appendicitis. However, different studies reported conflicting outcomes regarding the effectiveness of ERAT in comparison with laparoscopic appendectomy (LA).
AIM
To compare the effectiveness of ERAT with LA.
METHODS
Randomized controlled trials (RCTs) and retrospective studies of ERAT for acute uncomplicated appendicitis were searched in PubMed, Cochrane Library, Web of Science, Embase database, China National Knowledge Infrastructure (CNKI), the WanFang Database, and Chinese Scientific Journals Database (VIP) from the establishment date to March 1 2021. Heterogeneity was assessed using the I-squared statistic. Pooled odds ratios (OR), weighted mean difference (WMD), and standard mean difference (SMD), with 95% confidence intervals (CI) were calculated through either fixed-effects or random-effects model. Sensitivity analysis was also performed. Publication bias was tested by Egger's test, and Begg’s test. The quality of included RCT were evaluated by the Jadad scale, while Newcastle-Ottawa scale is adopted for assessing the methodological quality of case-control studies. All statistical analysis was performed using Stata 15.1 statistical software. All statistical analysis was performed using Stata 15.1 statistical software. This study is registered with PROSPERO, CRD42021243955.
RESULTS
After screening, 10 RCTs and 2 case-control studies were included in the current systematic review. Firstly, the length of hospitalizations [WMD = -1.15, 95%CI: -1.99, -0.31; P = 0.007] was shorter than LA group. Secondly, the level of post-operative CRP [WMD = -10.06, 95%CI: (-17.39, -2.73); P = 0.007], TNF-α [WMD = -7.70, 95%CI: (-8.47, -6.93); P < 0.001], and IL-6 Levels [WMD = -9.78, 95%CI: (-10.69, -8.88); P < 0.001; P < 0.001] in ERAT group was significantly lower than LA group. Thirdly, ERAT group had a lower incidence of intestinal obstruction than LA group. [OR = 0.19, 95%CI: (0.05, 0.79); P = 0.020]. Moreover, the quality of 10 RCTs were low with 0-3 Jadad scores, while the methodological quality of two case-control studies were fair with a score of 2 (each).
CONCLUSION
Compared with LA, ERAT reduces operation time, the level of postoperative inflammation, and results in fewer complications and shorter recovery time, with preserving the appendix and its immune and biological functions.
Keywords: Endoscopic retrograde appendicitis therapy, Acute appendicitis, Meta analysis, Laparoscopic appendectomy, Randomized controlled study
Core Tip: Acute appendicitis is one of the common surgical emergencies all over the world, with a mean cost of about $9000 per procedure. It is recognized that the conventional treatment of acute appendicitis was laparoscopic appendectomy (LA), while an increasing number of surgical complications, include bleeding, adhesive intestinal obstruction, infection of the incision, and intestinal fistula, have been reported. Therefore, we conducted a meta-analysis to compare the effectiveness of endoscopic retrograde appendicitis therapy (ERAT) with standard treatment. After screening, 12 studies were included in the current systematic review and we found that, compared with LA, ERAT reduces operation time, the level of postoperative inflammation, and results in fewer complications and shorter recovery time, with preserving the appendix and its immune and biological functions.
INTRODUCTION
Acute appendicitis is one of the common surgical emergencies all over the world, with a mean cost of about $9000 per procedure[1,2]. Appendicitis is one of the most frequent specific underlying causes in patients presenting to emergency departments with abdominal pain[3,4]. The majority (approximately 70%-80%) of acute appendicitis cases are of uncomplicated nature[5,6]. It is reported that the incidence of appendicitis is rising, which is about 1 per 1,000 in the America[7,8]. At present, the etiology of acute appendicitis is still unknown. Common etiological factors, including luminal obstruction from appendiceal fecalith, stool, lymphoid hyperplasia, and neoplasm result in about half of the cases, with stool and appendiceal fecalith as more common causes[9].
LA is currently widely applied for the treatment of acute appendicitis. Although patients could benefit from LA with a decreased wound infection rate, shorter hospital stay, and better diagnostic power[10], some complications can not be ignored. Liang TJ et al[11] investigated 864 patients who developed acute appendicitis recurrence in a median follow-up of 6.5 years. The authors found that 258 patients were performed LA, which accounted for about 30%. What’s more, an increasing number of surgical complications after LA , including bleeding, adhesive intestinal obstruction, infection of the incision, appendiceal remnants, and intestinal fistula[12].
In 2012, Liu et al[13] proposed a new endoscopic minimally invasive treatment for appendicitis, namely Endoscopic retrograde appendicitis therapy (ERAT). After preoperative bowel preparation, the appendix was intubated through the colonoscopy with a transparent cap at the head end, and the diagnosis of appendicitis was confirmed by angiography under X-ray monitoring. It can also relieve the obstruction of the appendix lumen, drain the pus, and flush the lumen to control the inflammation. It also allows the placement of drainage tube into the lumen to ensure the smooth drainage through the appendiceal orifice, reduce the risk of recurrence of appendicitis caused by obstruction.
Previous studies had shown ERAT as an effective treatment for acute appendicitis complicated with local perforation and/or periappendiceal abscess[14]. However, different studies reported conflicting outcomes regarding the effectiveness of ERAT in comparison with LA. Therefore, we conducted a meta-analysis to compare the effectiveness of ERAT with LA for adults.
MATERIALS AND METHODS
Preferred reporting items for systematic reviews and meta-analyses
The Preferred Reporting Items declared by the Systematic Review and Meta-Analysis (PRISMA)[15] was utilized in the performance of this study. The databases including PubMed, Cochrane Library, Web of Science, Embase database, China National Knowledge Infrastructure (CNKI), the WanFang Database, and Chinese Scientific Journals Database (VIP), were searched by using the searching terms including acute appendicitis (acute uncomplicated appendicitis) and endoscopic retrograde appendicitis therapy [endoscopic retrograde appendiceal radiography (ERAR), endoscopic appendiceal irrigation (EAI), and endoscopic appendiceal stent placement (ERSP)]. By taking the retrieval in PubMed as an example, the concrete retrieval strategies are as follows: (acute appendicitis [Mesh Terms] OR acute appendicitis [Title/Abstract] OR acute uncomplicated appendicitis[Mesh Terms] OR acute uncomplicated appendicitis [Title/Abstract]) AND (endoscopic retrograde appendicitis therapy [Mesh Terms] OR endoscopic retrograde appendicitis therapy [Title/Abstract] OR endoscopic retrograde appendiceal radiography [Mesh Terms] OR endoscopic appendiceal irrigation [Title/Abstract] OR endoscopic appendiceal stent placement [Title/Abstract]).
The retrieval time of each database is from the establishment of the database to March 1, 2021. The reference of related literatures and reviews were also retrieved manually to ensure that there was no omission, and the prospective study of ERAT on acute appendicitis published in the literatures are statistically analyzed. The protocol of this systematic review and meta-analysis has already prospectively registered in the PROSPERO (International Prospective Register of Systematic Reviews) database (reference no. CRD42021243955).
Study selection
Studies that met the following criteria were considered to be eligible for inclusion: (1) Study design: Randomized controlled trials, retrospective studies, and prospective studies; (2) Patients: The subjects were clinically diagnosed as acute uncomplicated appendicitis patients; (3) Outcomes: Literatures should provide accurate comprehensive statistical indicators: Sample Size, length of hospitalizations, operation time, recovery time, length of hospitalization, risk of complications; (4) Intervention and control: Intervention was endoscopic retrograde appendicitis therapy, while control group receiving LA; and (5) Articles published in English or Chinese. Exclusion criteria: (1) Duplicate publications; (2) Studies without sufficient data; and (3) Care reports, meta-analysis and reviews, study without English abstract and studies only with abstract were also excluded.
Literature quality evaluation and data extraction
Literature screened by two reviewers independently according to the inclusion and exclusion criteria mentioned above. Any disagreements were resolved through discussion with a third reviewer to reach a consensus. The following data were extracted: first author's name, the time of publication, the type of appendicitis, the participants of the experimental and control group, interventions, and outcomes (the bed rest time, time interval of body temperature returning to normal range, and time interval of white blood cell count returning to normal range, et al). Included RCT studies were evaluated by the Jadad scale regarding quality and methodology, where a higher score (total score of seven) suggests more rigorousness of a trial’s methodological design[16]. For both case-control and cohort studies, Newcastle-Ottawa scale[17] is adopted for assessing the methodological quality, which provides a comprehensive score system with eight items.
Statistical analysis
Heterogeneity test was performed with Stata 15.0 statistical software (Stata Corp., College Station, TX). The bed rest time, body temperature return to normal time and white blood cells return to normal time were combined by standard mean difference (SMD) with 95%CI, while duration of operation, length of hospitalizations, and levels of inflammatory factors were combined by weighted mean difference (WMD) with 95%CI. Q-test and I2-test were used to analyze the heterogeneity of the studies included in this meta-analysis. If P > 0.100 and I2 < 50%, it was considered that there was small heterogeneity among the studies, and fixed effect model was chosen; otherwise, random effect model was used to merge SMD with 95%CI[18]. The pooled relative risk (RR) with 95%CI: Was performed to analyze the risk of complications. Data of the outcomes were recorded for this meta-analysis when three or more trials reported the same outcome. Sensitivity analyses were performed to investigate the robustness of this meta-analysis. Meanwhile, the risk of publication bias was evaluated by Egger’s test, Begg’s test, and funnel plots[19]. If the heterogeneity shown P < 0.100 and I2 > 50%, considered that there was large heterogeneity among the studies. Egger’s test was assessed by using Stata 15.0.
RESULTS
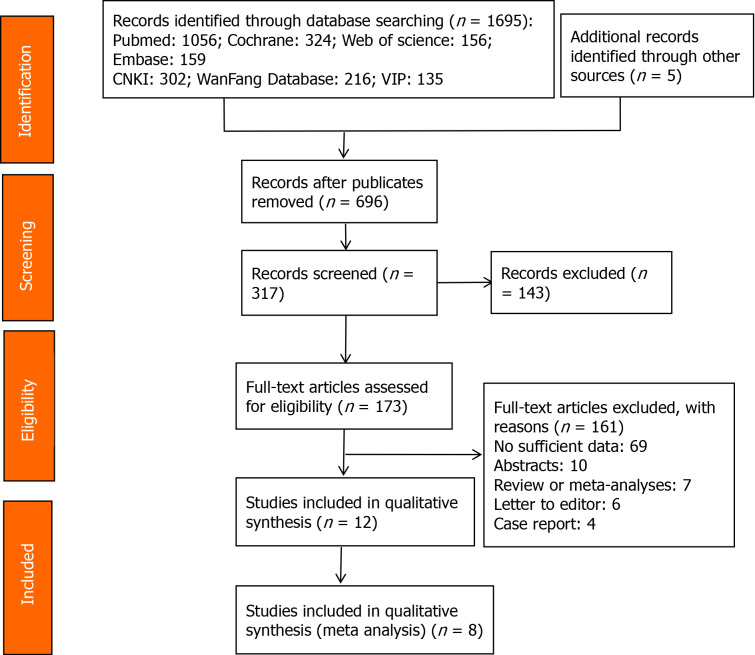
From the 1,013 relevant records initially identified, 696 remained after excluding duplicates. Then 143 articles were excluded after subsequent scanning of the titles and abstracts. Full texts of the 161 records remained were scrutinized, and 12 studies[20-31] that met the inclusion criteria were selected in systematic review, while 8 studies[21-24,26,28,30,31] were included in meta analysis. The flow of selecting included studies was shown in Figure 1. The 12 included articles with 970 subjects were published between 2016 and 2020 and included 2 case-control[27,31] studies, and 10 RCTs. More detailed characteristics were summarized in Table 1. The Jadad scores of 10 included studies were 0-3 scores. Meanwhile, the methodological quality of two case-control studies[27,31] were fair, with a score of 2 (each). The Jadad score of included studies were shown in Table 2 and Newcastle-Ottawa scale score was shown in Sup plementary Table 1.
Figure 1.
Flow diagram representing the selection of studies.
Table 1.
Detailed characteristics of included studies in this meta analysis
|
Ref.
|
Studies types |
Patients age
|
Treatment
|
Sample size
|
Disease | Outcomes | ||
|
Experiment
|
Control
|
Experiment
|
Control
|
|||||
| Kang et al[20], 2020 | RCT | 1 to 13 years old | Modified ERAT | Antibiotics treatment | 36 | 47 | Acute uncomplicated appendicitis in children | Length of hospital stay |
| Deng et al[21], 2018 | RCT | 18-62 years old | ERAT | Laparoscopic appendectomy | 20 | 20 | Acute appendicitis | Duration of operation, Bed rest time; time interval of body temperature returning to normal range; time interval of white blood cells count returning to normal time range, complication |
| Huang et al[22], 2020 | RCT | 18-65 years old | ERAT | Laparoscopic appendectomy | 78 | 119 | Acute appendicitis | Duration of operation, bed rest time, complication |
| Lin et al[23], 2016 | RCT | 18-70 years old | ERAT | Laparoscopic appendectomy/antibiotics treatment | 44 | 45/36 | Simple appendicitis | Length of hospital stay, bed rest time, time interval of body temperature returning to normal range, inflammatory factors, complication |
| Ma et al[24], 2020 | RCT | 19-74 years old | ERAT | Laparoscopic appendectomy | 20 | 20 | Non-complex appendicitis | Duration of operation, length of hospital stay, time interval of body temperature returning to normal range, inflammatory factors, complication |
| Wang et al[25], 2017 | RCT | 3 to 13 years old | ERAT | Laparoscopic appendectomy | 42 | 42 | Acute uncomplicated appendicitis in children | Duration of operation, length of hospital stay, bed rest time, time interval of body temperature returning to normal range, complication |
| Pan et al[26], 2018 | RCT | 19-62 years old | ERAT | Laparoscopic appendectomy | 35 | 36 | Acute appendicitis | Duration of operation, length of hospital stay, bed rest time, inflammatory factors |
| Shen et al[27], 2020 | Case-control | NA | ERAT combined with antibiotics treatment | Antibiotics treatment | 42 | 57 | Acute appendicitis | Length of hospital stay |
| Ye et al[28], 2016 | RCT | 18-70 years old | ERAT | Laparoscopic appendectomy | 57 | 57 | Non-perforated acute appendicitis | Length of hospital stay, bed rest time, inflammatory factors, complication |
| Zhu et al[29], 2018 | RCT | NA | ERAT | Antibiotics treatment | 17 | 24 | Atypical acute appendicitis | Complication |
| Yang et al[30], 2016 | RCT | 20-60 years old | ERAT | Laparoscopic appendectomy | 35 | 35 | Acute uncomplicated appendicitis | Duration of operation, bed rest time, length of hospital stay, time interval of body temperature returning to normal range |
| Li et al[31], 2016 | Case-control | 14-73 years old | ERAT | Laparoscopic appendectomy | 21 | 20 | Uncomplicated acute appendicitis | Duration of operation, length of hospital stay, bed rest time, time interval of body temperature returning to normal range, time interval of white blood cells count returning to normal time range, complication |
ERAT: Endoscopic retrograde appendicitis therapy.
Table 2.
Detailed quality assessment of included studies using modified Jadad score
|
Ref.
|
Randomization
|
Concealment of allocation
|
Double blinding
|
Description of withdrawals and dropouts
|
Total score
|
| Kang et al[1], 2018 | 2 | 0 | 0 | 1 | 3 |
| Deng et al[2], 2018 | 0 | 0 | 0 | 1 | 1 |
| Huang et al[3], 2020 | 2 | 0 | 0 | 0 | 0 |
| Lin et al[4], 2016 | 0 | 0 | 0 | 0 | 0 |
| Ma et al[5], 2020 | 1 | 0 | 0 | 0 | 1 |
| Wang et al[6], 2017 | 2 | 0 | 0 | 0 | 2 |
| Pan et al[7], 2018 | 2 | 0 | 0 | 0 | 2 |
| Wu et al[9], 2019 | 0 | 0 | 0 | 1 | 1 |
| Ye et al[10], 2016 | 0 | 0 | 0 | 1 | 1 |
| Zhang et al[11], 2017 | 1 | 0 | 0 | 0 | 1 |
| Zhu et al[12], 2018 | 2 | 0 | 0 | 1 | 3 |
| Yang et al[13], 2016 | 2 | 0 | 0 | 0 | 2 |
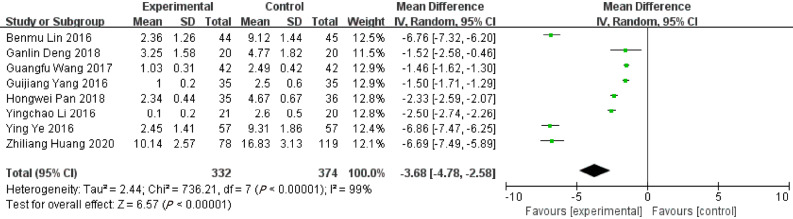
Bed rest time
Eight records reported the bed rest time in ERAT group and LA group. The bed rest time in ERAT group was shorter than LA group [WMD = -3.68, 95%CI: (-4.78, -2.58); P < 0.001], with high heterogeneity [Q = 736.21, P heterogeneity < 0.001, I2 = 99.0%]. Shown in Figure 2.
Figure 2.
Forest plot of bed rest time.
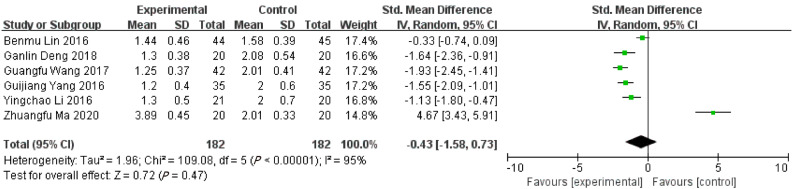
Time interval of body temperature returning to normal range
The time interval of body temperature returning to normal range in ERAT group was shorter than LA group based on 6 included studies. [SMD = -0.43, 95%CI: (-1.58, 0.73); P = 0.481] with high heterogeneity [Q = 113.64, P heterogeneity < 0.001, I2 = 95.6%]. Shown in Figure 3.
Figure 3.
Forest plot of time interval of body temperature returning to normal range.
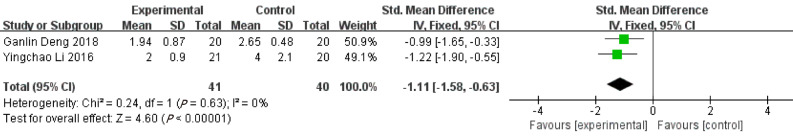
Time interval of white blood cell count returning to normal range
Based on 2 included studies, the time interval of leukocyte count returning to normal range in patients receiving ERAT group was shorter than that in LA group [SMD = -1.11, 95%CI: (-1.58, -0.63); P < 0.001] with low heterogeneity [Q = 0.24, P heterogeneity = 0.630, I2 = 0.00%]. See Figure 4.
Figure 4.
Forest plot of time interval of white blood cell count returning to normal range.
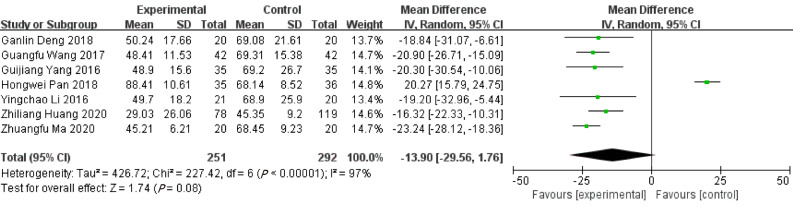
Duration of operation
Seven studies reported the duration of ERAT in comparison to LA. There was no difference regarding duration of operation between ERAT group and LA group [WMD = -13.90, 95%CI: (-29.56, 1.76); P = 0.08] with high heterogeneity [Q = 227.42, P heterogeneity < 0.001, I2 = 97.4%)]. Shown in Figure 5.
Figure 5.
Forest plot of duration of operation.
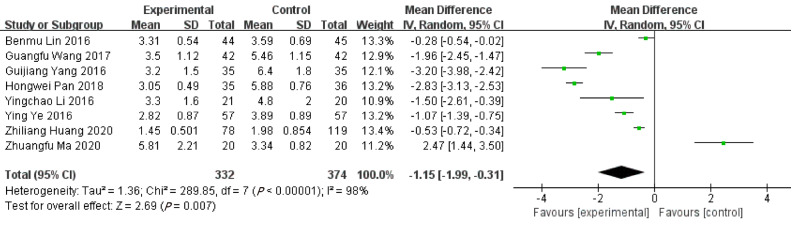
Length of hospitalizations
Based on 8 included studies, the length of hospitalizations in ERAT group was shorter than LA group. [WMD = -1.15, 95%CI: (-1.99, -0.31); P = 0.007] with high heterogeneity [Q = 289.85, P heterogeneity < 0.001, I2 = 97.6%]. Shown in Figure 6.
Figure 6.
Forest plot of length of hospitalizations.
Levels of inflammatory factors
C-reactive protein (CRP): Based on 3 included studies[24,26,28], there was no difference of pre-operative CRP levels between ERAT group and LA group [WMD = -0.28, 95%CI: (-1.14, 0.58); P = 0.53] with high heterogeneity [Q = 7.21, P heterogeneity = 0.03, I2 = 72.0%]. However, the level of post-operative CRP in ERAT group was significantly lower than that in LA group. [WMD = -10.06, 95%CI: (-17.39, -2.73); P = 0.007] with high heterogeneity [Q = 109.28, P heterogeneity < 0.001, I2 = 98.0%). Shown in Table 3.
Table 3.
Pooled results of inflammatory factors and complications
|
Outcomes
|
Categories
|
Number of records
|
OR/WMD and 95%CI
|
P
|
Heterogeneity with groups (I2)
|
Phet
value
|
| Inflammatory factors | ||||||
| C-reactive protein (pre) | 3 | -0.28, [-1.14, 0.58] | 0.53 | 72% | 0.03 | |
| C-reactive protein (post) | 3 | -10.06, [-17.39, -2.73] | 0.007 | 98.0% | < 0.001 | |
| Tumor necrosis factor-α (pre) | 2 | -0.21, [-1.32, 0.90] | 0.71 | 0.0% | 0.68 | |
| Tumor necrosis factor-α (post) | 2 | 7.70, [-8.47, -6.93] | < 0.001 | 99.0% | < 0.001 | |
| Interleukin 6 (pre) | 3 | -0.11, [-1.04, 0.82] | 0.81 | 6.0% | 0.34 | |
| Interleukin 6 (post) | 3 | -9.78, [-10.69, -8.88] | < 0.001 | 99.0% | < 0.001 | |
| Complications | ||||||
| Intestinal obstruction | 4 | 0.19, [0.05, 0.79] | 0.020 | 0.0% | 0.95 | |
| Abdominal infection | 2 | 0.10, [0.01, 0.83] | 0.030 | 0.0% | 0.44 | |
| Urinary tract infection | 3 | 0.27, [0.04, 1.65] | 0.160 | 0.0% | 0.97 |
Tumor necrosis factor-α (TNF-α): Based on 2 included studies[24,26], there was no difference of pre-operative levels of TNF-α between ERAT group and LA group [WMD = -0.21, 95%CI: (-1.32, 0.90); P = 0.71] with low heterogeneity [Q = 0.17, P heterogeneity = 0.68, I2 = 0.00%]. However, the level of TNF-α in ERAT group was significantly lower than LA group after operating. [WMD = -7.70, 95%CI: (-8.47, -6.93); P < 0.001] with high heterogeneity [Q = 138.67, P heterogeneity < 0.001, I2 = 99.0%). Shown in Table 3.
Interleukin 6 (IL-6): Based on 3 included studies[24,26,28], no difference of pre-operative levels of IL-6 was found between ERAT group and LA group [WMD = -0.11, 95%CI: (-1.04, 0.82); P = 0.81] with low heterogeneity [Q = 2.13, P heterogeneity = 0.34, I2 = 6.0%]. However, the level of IL-6 in ERAT group was significantly lower than LA group, post-operatively. [WMD = -9.78, 95%CI: (-10.69, -8.88); P < 0.001] with high heterogeneity [Q = 163.52, P heterogeneity < 0.001, I2 = 99.0%). Shown in Table 3.
Complications
Intestinal obstruction: Four studies[22,24,28,31] reported the intestinal obstruction after operation. The pooled result shown that ERAT group had a lower incidence of intestinal obstruction than LA group. [OR = 0.19, 95%CI: (0.05, 0.79); P = 0.020] with low heterogeneity [Q = 0.34, P heterogeneity = 0.95, I2 = 0.00%]. Shown in Table 3.
Abdominal infection: Two studies[24,31] reported the abdominal infection after operation. The pooled result found that ERAT group had a lower incidence of abdominal infection than LA group [OR = 0.10, 95%CI: (0.01, 0.83); P = 0.350] with low heterogeneity [Q = 0.60, P heterogeneity = 0.44, I2 = 0.00%]. Shown in Table 3.
Urinary tract infection (UTI): The pooled result of 3 studies[25,28,31] reporting post-operative UTI did not find statistically significant difference between ERAT group and LA group [OR = 0.27, 95%CI: (0.04, 1.65); P = 0.160] with low heterogeneity [Q = 0.07, P heterogeneity = 0.97, I2 = 0.00%]. Shown in Table 3.
Sensitivity analysis
Furtherly, sensitivity analysis was performed to investigate the robustness of this meta-analysis. The results of sensitivity analysis shown that one study had a significant influence on the result of duration of operation[26], one study had a significant influence on the result of time interval of body temperature returning to the normal range[23], one study had a significant influence on the result of CRP (post-operative)[26], no study had a significant influence on the result of TNF (pre-operative) and one study had a significant influence on the result of IL-6 (pre-operative) [28].
Bias analysis
No obvious publication bias was depicted by the funnel plot (Supplementary Figure 1) and result from Egger’s test (t = -0.06, P = 0.954) and Begg’s test (Z = 0.30, P = 0.764) indicated no evidence of publication bias with regard to the duration of the operation. All outcomes of bias analysis were shown in Table 4.
Table 4.
Publication bias of outcomes by Egger’s test and Begg’s test
|
Egger’s test
|
Begg’s test
|
|||
|
t
|
P
|
Z
|
P
|
|
| Time interval of body temperature returning to normal rangetime | 1.17 | 0.306 | 0.75 | 0.452 |
| Time interval of White white blood cells count returning to normal timerange | - | - | 0.00 | 1.00 |
| Duration of operation | -0.9 | 0.409 | 1.2 | 0.230 |
| Length of hospitalizations (vs LA) | -0.48 | 0.648 | 0.37 | 0.711 |
| Length of hospitalizations (vs Anti) | -1.72 | 0.336 | 0.00 | 1.00 |
| CRP (pre-operative) | 2.23 | 0.268 | 1.04 | 0.296 |
| CRP (post-operative) | -0.19 | 0.878 | 0.00 | 1.00 |
| TNF-α (pre-operative) | - | - | 0.00 | 1.00 |
| TNF-α (post-operative) | - | - | 0.00 | 1.00 |
| IL-6 (pre-operative) | -1.27 | 0.425 | 0.00 | 1.00 |
| IL-6 (post-operative) | -7.43 | 0.085 | 1.04 | 0.296 |
| Intestinal obstruction | 2.03 | 0.179 | 1.70 | 0.089 |
| Abdominal infection | - | - | 0.00 | 1.00 |
| Urinary tract infection | 11.87 | 0.053 | 0.00 | 1.00 |
| Bed rest time | -3.1 | 0.021 | 1.11 | 0.266 |
LA: Laparoscopic appendectomy.
DISCUSSION
Acute appendicitis, as one of the common surgical diseases, is the most common causes of surgical acute abdomen[32]. The latest study reported that the morbidity of acute appendicitis is as high as 6% in the population[33]. It has been found that the appendix can secrete a variety of useful substances and hormones (such as digestive enzymes, hormones that promote intestinal peristalsis, hormones related to growth), and play immune function to resist various diseases[34]. In addition, as the appendix contains a variety of intestinal microorganisms, it plays a key role in maintaining the balance of intestinal flora[35]. At present, the treatment for acute non-complex appendicitis includes surgery and conservative antibiotic treatment[36]. In order to preserve the potentially important function of the appendix, a retrograde endoscopic appendicitis treatment for acute simple appendicitis was first proposed in 2012. ERAT has the advantages of convenient operation, small trauma, and rapid relief of pain after the pressure of the appendix cavity is lifted[37]. In order to explore the safety of ERAT and provide more evidence for clinical treatment, this meta-analysis was conducted to investigate postoperative complications, length of hospitalizations, operation time, postoperative bed rest time, and indicators of recovery. The results showed that ERAT had shorter time intervals of white blood cell count returning to normal range, length of hospitalizations, and bed rest time. Meanwhile, the incidence of complications is lower, and the postoperative recovery time is faster compared with LA.
In 2008, Mason et al[38] proposed that about 70% of patients with acute appendicitis do not need appendectomy and can be treated conservatively. Recently, Prechal et al[39] pointed out in a meta-analysis that appendectomy is more effective than antibiotic treatment in the treatment of acute uncomplicated appendicitis, and that the incidence of complications of the two treatment schemes is almost the same. Although ERAT emerges recently as a relatively new modality of treatment, it shows unique advantages. The latest research reported by Liu et al[18], the abdominal pain of 32 acute uncomplicated appendicitis patients resolved immediately after ERAT operation, and the clinical success rate was 97%. Colonoscopic irrigation, as a type of ERAT, was performed on 10 patients with acute appendicitis by Feng Jia et al[40]. Follow-up results found that there was no tenderness in the abdomen on physical examination, and no fever and other symptoms after operation. Notably, during the follow-up period of 1-8 mo, no complications occurred, and 9 cases had no recurrence of appendicitis. Chen et al[41] performed ERAT on 101 patients with acute appendicitis, the results showed that the success rate of appendiceal intubation was 96% (97/101), the success rate of treatment was 97.9% (94/96). Meanwhile, the operation time, the temperature recovery time, the white blood cell recovery time, and the abdominal pain relief time was shorter than the control group. What is more, no postoperative complications were detected. In addition, regarding the complication after ERAT, Li Yingchao et al[31] compared ERAT with LA and the results showed that perforation occurred in 1 case (5%) in ERAT group, and complications occurred in 3 cases (15%) in LA group. After more than half a year of follow-up, 2 cases in ERAT group were highly suspected of "chronic appendicitis" (recurrence rate 2/20, 10%), while no recurrence of appendicitis in LA group was reported, however, during a follow-up period of at least six months after surgery, 10 cases in LA group had postoperative diarrhea and constipation. Conversely, the results from Deng Ganlin et al[21] showed that the incidence of postoperative complications of the ERAT group was lower than that of the LA group, but the difference was not statistically significant (P > 0.05). Ma Zhuangfu et al[24] found that 1 sary intestinal obstruction occurred in ERAT group, while 6 sary intestinal obstructions occurred in LA group. Notably, our study shown that ERAT group had a lower incidence of intestinal obstruction than LA group based on 7 included studies. Lin et al[23] found that no patients with UTI and abdominal infection after ERAT, while 2 patients with UTI and 1 patient with abdominal infection were discovered in LA group, while this comprehensive meta-analysis demonstrated that there was no difference between ERAT group and control group regarding abdominal infection and UTI.
The serum inflammatory factors of the patients between ERAT and control group were analyzed by Pan Hongwei[26], and the results showed the serum levels of hypersensitive CRP, IL-6, and TNF-α between ERAT group and LA group were significantly decreased after operation compared with those before operation, and the ERAT group was lower than the control group; The serum levels of hypersensitive CRP, IL-6, and TNF-α in the two groups were significantly decreased after operation compared with those before operation, and the ERAT group was lower than the control group (P < 0.05). CRP is an acute response protein secreted by the liver, and is also an essential inflammatory medium[42] to measure the intensity of response to trauma. IL-1β, TNF-α, and IL-6 are common pro-inflammatory factors, and their secretion is increased in both acute and chronic inflammation, jointly promoting multiple pathological injury processes such as tissue destruction and edema formation[43,44]. IL-6 is also a typical pro-inflammatory factor, produced by activated T cells and fibroblasts, and can cooperatively activate inflammation-related signals with TNF-α to induce cascade reaction[45] and induce the production of other pro-inflammatory factors[46]. It is a common anti-inflammatory factor and has the effect of reducing inflammatory cell overactivation[47]. Therefore, we conducted the pooled analysis of these markers which shown that there was no difference in pre-operative levels of TNF-α, IL-6, and CRP between ERAT group and LA group, while the level of TNF-α, IL-6, and CRP in ERAT group was significantly lower than LA group after operating. However, we acknowledge that the timing of post-ERAT measurement of inflammatory factors is various across included studies, which may be one of the sources of heterogeneity.
Appendectomy has long been the most important method for the treatment of acute appendicitis. Although LA has faster recovery, less pain, and less wound infection compared with open surgery[48,49], there is still a certain risk of postoperative complications, and it has been reported[50,51] that the negative resection rate of appendix is as high as 8%-15%. Based on our meta-analysis, it is found that ERAT has its own unique advantages of being faster, more effective, and safer, compared with LA.
Limitation
First, the high heterogeneity across included studies was found, which could be attributed to different severities of the patients enrolled in each study, different mean ages of each study, different operating experience of ERAT of gastroenterologists and endoscopists in each study, and different study designs. Second, as little study compared LA with antibiotics treatment as well as compared adults with children, it is difficult to perform a meta-analysis regarding these outcomes. Third, limited studies were reported in other areas outside China.
CONCLUSION
Compared with LA treatment, ERAT reduces operation time, and results in fewer complications and shorter recovery time, with preserving the appendix and its immune and biological functions. However, given that only a limited number of studies were reported and most were conducted in China, more original studies with high quality in multi-centers from different countries and areas are still needed to further explore this novel modality of treatment for appendectomy.
ARTICLE HIGHLIGHTS
Research background
Evidence from revious studies shown that endoscopic retrograde appendicitis therapy (ERAT) is an effective treatment for acute appendicitis.
Research motivation
However, different studies reported conflicting outcomes regarding the effectiveness of ERAT in comparison with laparoscopic appendectomy (LA).
Research objectives
This meta-analysis was conducted to compare the effectiveness of ERAT with LA.
Research methods
Randomized controlled trials and retrospective studies of ERAT for acute uncomplicated appendicitis were searched in PubMed, Cochrane Library, Web of Science, Embase database, China National Knowledge Infrastructure (CNKI), the WanFang Database, and Chinese Scientific Journals Database (VIP).
Research results
10 randomized controlled studies (RCTs) and 2 case-control studies were included in the current systematic review. Firstly, the length of hospitalizations [WMD = -1.15, 95%CI: (-1.99, -0.31); P = 0.007] was shorter than LA group. Secondly, the level of post-operative CRP [WMD = -10.06, 95%CI: (-17.39, -2.73); P = 0.007], TNF-α [WMD = -7.70, 95%CI: (-8.47, -6.93); P < 0.001], and IL-6 Levels [WMD = -9.78, 95%CI: (-10.69, -8.88); P < 0.001; P < 0.001] in ERAT group was significantly lower than LA group. Thirdly, ERAT group had a lower incidence of intestinal obstruction than LA group. [OR = 0.19, 95%CI: (0.05, 0.79); P = 0.020].
Research conclusions
Based on our meta-analysis, it is found that ERAT has its own unique advantages of being more effective, safer compared with LA.
Research perspectives
As little study compared LA with antibiotics treatment, future study should focus on comparing the effectiveness between LA and antibiotics treatment.
Footnotes
Conflict-of-interest statement: Allauthors have no conflict(s) of interest to declare in relation to this manuscript.
PRISMA 2009 Checklist statement: The authors have read the PRISMA 2009 Checklist, and the manuscript was prepared and revised in accordance with this checklist.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review started: June 5, 2021
First decision: June 27, 2021
Article in press: October 14, 2021
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosseini MS, Maslennikov R S-Editor: Wang LL L-Editor: A P-Editor: Wang LL
Contributor Information
Ying Wang, Department of Endoscopy Center, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Chen-Yu Sun, Internal Medicine, AMITA Health Saint Joseph Hospital Chicago, Chicago, IL 60657, United States.
Jie Liu, Department of Gastroenterology, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
Yue Chen, Department of Clinical Medicine, School of the First Clinical Medicine, Anhui Medical University, Hefei 230032, Anhui Province, China.
Chandur Bhan, Internal Medicine, AMITA Health Saint Joseph Hospital Chicago, Chicago, IL 60657, United States.
John Pocholo Whitaker Tuason, Internal Medicine, AMITA Health Saint Joseph Hospital Chicago, Chicago, IL 60657, United States.
Sudha Misra, Internal Medicine, AMITA Health Saint Joseph Hospital Chicago, Chicago, IL 60657, United States.
Yu-Ting Huang, University of Maryland Medical Center Midtown Campus, Baltimore, MD 21201, United States.
Shao-Di Ma, Department of Epidemiology and Health Statistics, School of Public Health Anhui Medical University, Hefei 230032, Anhui Province, China.
Xing-Yu Cheng, Department of Clinical Medicine, School of the First Clinical Medicine, Anhui Medical University, Hefei 230032, Anhui Province, China.
Qin Zhou, Department of Radiation Oncology, Mayo Clinic, Rochester, MN 55905, United States.
Wen-Chao Gu, Department of Diagnostic Radiology and Nuclear Medicine, Gunma University Graduate School of Medicine, Maebashi 371-8511, Japan.
Dan-Dan Wu, Department of Endoscopy Center, The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China. 16013255@qq.com.
Xia Chen, Department of Nursing,The First Affiliated Hospital of USTC, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei 230001, Anhui Province, China.
References
- 1.Alore EA, Ward JL, Rob TS. Population-level outcomes of early versus delayed appendectomy for acute appendicitis using the American College of Surgeons National Surgical Quality Improvement Program. J Surg Res . 2018:229: 234–242. doi: 10.1016/j.jss.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 2.Rentea RM, Peter SDS, Snyder CL. Pediatric appendicitis: state of the art review. Pediatr Surg Int . 2017;33:269–283. doi: 10.1007/s00383-016-3990-2. [DOI] [PubMed] [Google Scholar]
- 3.Fagerström A, Paajanen P, Saarelainen H, Ahonen-Siirtola M, Ukkonen M, Miettinen P, Paajanen H. Non-specific abdominal pain remains as the most common reason for acute abdomen: 26-year retrospective audit in one emergency unit. Scand J Gastroenterol . 2017;52:1072–1077. doi: 10.1080/00365521.2017.1342140. [DOI] [PubMed] [Google Scholar]
- 4.Georgiou R, Eaton S, Stanton MP, Pierro A, Hall NJ. Efficacy and Safety of Nonoperative Treatment for Acute Appendicitis: A Meta-analysis. Pediatrics . 2017;139 doi: 10.1542/peds.2016-3003. [DOI] [PubMed] [Google Scholar]
- 5.Drake FT, Mottey NE, Farrokhi ET, Florence MG, Johnson MG, Mock C, Steele SR, Thirlby RC, Flum DR. Time to appendectomy and risk of perforation in acute appendicitis. JAMA Surg . 2014;149:837–844. doi: 10.1001/jamasurg.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Körner H, Söndenaa K, Söreide JA, Andersen E, Nysted A, Lende TH, Kjellevold KH. Incidence of acute nonperforated and perforated appendicitis: age-specific and sex-specific analysis. World J Surg . 1997;21:313–317. doi: 10.1007/s002689900235. [DOI] [PubMed] [Google Scholar]
- 7.Buckius MT, McGrath B, Monk J, Grim R, Bell T, Ahuja V. Changing epidemiology of acute appendicitis in the United States: study period 1993-2008. J Surg Res . 2012;175:185–190. doi: 10.1016/j.jss.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 8.Anderson JE, Bickler SW, Chang DC, Talamini MA. Examining a common disease with unknown etiology: trends in epidemiology and surgical management of appendicitis in California, 1995-2009. World J Surg . 2012;36:2787–2794. doi: 10.1007/s00268-012-1749-z. [DOI] [PubMed] [Google Scholar]
- 9.Sanders NL, Bollinger RR, Lee R, Thomas S, Parker W. Appendectomy and Clostridium difficile colitis: relationships revealed by clinical observations and immunology. World J Gastroenterol . 2013;19:5607–5614. doi: 10.3748/wjg.v19.i34.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moberg AC, Ahlberg G, Leijonmarck CE, Montgomery A, Reiertsen O, Rosseland AR, Stoerksson R. Diagnostic laparoscopy in 1043 patients with suspected acute appendicitis. Eur J Surg . 1998;164:833–40; discussion 841. doi: 10.1080/110241598750005246. [DOI] [PubMed] [Google Scholar]
- 11.Liang TJ, Liu SI, Tsai CY, Kang CH, Huang WC, Chang HT, Chen IS. Analysis of Recurrence Management in Patients Who Underwent Nonsurgical Treatment for Acute Appendicitis. Medicine (Baltimore) . 2016;95:e3159. doi: 10.1097/MD.0000000000003159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang L, Yin Y, Yang L, Wang C, Li Y, Zhou Z. Comparison of Antibiotic Therapy and Appendectomy for Acute Uncomplicated Appendicitis in Children: A Meta-analysis. JAMA Pediatr . 2017;171:426–434. doi: 10.1001/jamapediatrics.2017.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu BR, Song JT, Han FY, Li H, Yin JB. Endoscopic retrograde appendicitis therapy: a pilot minimally invasive technique (with videos) Gastrointest Endosc . 2012;76:862–866. doi: 10.1016/j.gie.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez DO, Deans KJ, Minneci PC. Role of non-operative management in pediatric appendicitis. Semin Pediatr Surg . 2016;25:204–207. doi: 10.1053/j.sempedsurg.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 15.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ . 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials . 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 17.The Newcastle-Ottawa Scale for Assessing the Quality if Nonrandomized Studies in MetaAnalyses. [cited 20 February 2021]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm .
- 18.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med . 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 19.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang J, Zhang W, Zeng L, Lin Y, Wu J, Zhang N, Xie X, Zhang Y, Liu X, Wang B, Yang R, Jiang X. The modified endoscopic retrograde appendicitis therapy vs antibiotic therapy alone for acute uncomplicated appendicitis in children. Surg Endosc . 2020 doi: 10.1007/s00464-020-08129-8. [DOI] [PubMed] [Google Scholar]
- 21.Deng GL, Ceng ZX, Chen LF. Effect of colonoscopy in the treatment of acute appendicitis. Hainan Yixue Zazhi . 2018;11:1594–1596. [Google Scholar]
- 22.Huang ZL, Huo ZH, Shu YM. Application of endoscopic retrograde appendicitis therapy in acute appendicitis. Hainan Yixue Zazhi . 2020;11:1028–1103. [Google Scholar]
- 23.Lin BM, Sun XZ, Liu YY. Comparison on the therapeutic effect of different treatments for simple appendicitis complicated with diabetes mellitus. Hainan Yixue Zazhi . 2016;19:3157–3160. [Google Scholar]
- 24.Ma ZF, Huang RW. Endoscopic retrograde appendicitis therapy in treatment of acute non-complex appendicitis. Zhongguo Neijing Zazhi . 2020;26:7–12. [Google Scholar]
- 25.Wang GF. Efficacy of endoscopic retrograde appendicitis in children with acute non perforated appendicitis. Yingxiang Yanjiu Yixue Yingyong Zazhi . 2017;18:230–231. [Google Scholar]
- 26.Pan HW, Weng JJ. Prevention value of endoscopic retrograde appendicitis treatment for postoperative with appendicitis. Zhonghua Xiaohua Neijing Zazhi . 2018;35:405–409. [Google Scholar]
- 27.Shen WY, Tang J, Wu T. Comparative study on the efficacy of conservative treatment and endoscopic retrograde appendicitis treatment for acute appendicitis. Hainan Yixue Zazhi . 2020;24:3208–3210. [Google Scholar]
- 28.Ye Y, Sun XZ, Yang LM. Application of endoscopic retrograde appendicitis therapy in non-perforated acute appendicitis. Zhongguo Linchuang Yanjiu Zazhi . 2016;29:741–745. [Google Scholar]
- 29.Zhu FY, Chen T, Fu Z. Diagnostic and therapeutic value of endoscopic retrograde appendicitis therapy for atypical acute appendicitis. Zhonghua Xiaohua Neijing Zazhi . 2018;35:571–575. [Google Scholar]
- 30.Yang GJ, Hu XF. The Value of Endoscopic Retrograde Appendicitis in the Treatment of Acute Uncomplicated Appendicitis. Zhongguo Jixu Yixue Jiaoyu . 2016;8:108–109. [Google Scholar]
- 31.Li YC, Mi C, Li WZ. Effect and safety of Endoscopic retrograde appendicitis therapy in treating patients with uncomplicated acute appendicitis. Zhongguo Neijing Zazhi . 2016;22:11–17. [Google Scholar]
- 32.Bhangu A, Søreide K, Di Saverio S, Assarsson JH, Drake FT. Acute appendicitis: modern understanding of pathogenesis, diagnosis, and management. Lancet . 2015;386:1278–1287. doi: 10.1016/S0140-6736(15)00275-5. [DOI] [PubMed] [Google Scholar]
- 33.Snyder MJ, Guthrie M, Cagle S. Acute Appendicitis: Efficient Diagnosis and Management. Am Fam Physician . 2018;98:25–33. [PubMed] [Google Scholar]
- 34.Chen SH, Yeong EK, Tang YB, Chen HC. Free and pedicled appendix transfer for various reconstructive procedures. Ann Plast Surg . 2012;69:602–606. doi: 10.1097/SAP.0b013e31827475e2. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Sali A, Vitetta L. The gallbladder and vermiform appendix influence the assemblage of intestinal microorganisms. Future Microbiol . 2020;15:541–555. doi: 10.2217/fmb-2019-0325. [DOI] [PubMed] [Google Scholar]
- 36.Coccolini F, Fugazzola P, Sartelli M, Cicuttin E, Sibilla MG, Leandro G, De' Angelis GL, Gaiani F, Di Mario F, Tomasoni M, Catena F, Ansaloni L. Conservative treatment of acute appendicitis. Acta Biomed . 2018;89:119–134. doi: 10.23750/abm.v89i9-S.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.LIU Bingrong, SONG Jitao, MA Xiao. Introduction of endoscopic retrograde appendicitis treatment technology. Zhonghua Xiaohua Neijing Zazhi . 2013;30:468. [Google Scholar]
- 38.Mason RJ. Surgery for appendicitis: is it necessary? Surg Infect (Larchmt) . 2008;9:481–488. doi: 10.1089/sur.2007.079. [DOI] [PubMed] [Google Scholar]
- 39.Podda M, Gerardi C, Cillara N, Fearnhead N, Gomes CA, Birindelli A, Mulliri A, Davies RJ, Di Saverio S. Antibiotic Treatment and Appendectomy for Uncomplicated Acute Appendicitis in Adults and Children: A Systematic Review and Meta-analysis. Ann Surg . 2019;270:1028–1040. doi: 10.1097/SLA.0000000000003225. [DOI] [PubMed] [Google Scholar]
- 40.FENG Jia, FENG Zitan, SUN Rong. Effect Observation of endoscopic intracavity douching in treatment of paitents with acute appendicitis[J] Jiefangjun Yixue Zazhi . 2014;26:46–47. [Google Scholar]
- 41.Chen Y, Wang M, Chen H, Zhao L, Liu L, Wang X, Huang J, Fan Z. WITHDRAWN: Endoscopic intervention for acute appendicitis: retrospective study of 101 cases. Gastrointest Endosc . 2019 doi: 10.1016/j.gie.2019.06.012. [DOI] [PubMed] [Google Scholar]
- 42.Trial J, Cieslik KA, Entman ML. Phosphocholine-containing ligands direct CRP induction of M2 macrophage polarization independent of T cell polarization: Implication for chronic inflammatory states. Immun Inflamm Dis . 2016;4:274–288. doi: 10.1002/iid3.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Broekman W, Amatngalim GD, de Mooij-Eijk Y, Oostendorp J, Roelofs H, Taube C, Stolk J, Hiemstra PS. TNF-α and IL-1β-activated human mesenchymal stromal cells increase airway epithelial wound healing in vitro via activation of the epidermal growth factor receptor. Respir Res . 2016;17:3. doi: 10.1186/s12931-015-0316-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang P, Wu X, Li G, He Q, Dai H, Ai C, Shi J. Tumor necrosis factor-alpha gene polymorphisms and susceptibility to ischemic heart disease: A systematic review and meta-analysis. Medicine (Baltimore) . 2017;96:e6569. doi: 10.1097/MD.0000000000006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chae JW, Ng T, Yeo HL, Shwe M, Gan YX, Ho HK, Chan A. Impact of TNF-α (rs1800629) and IL-6 (rs1800795) Polymorphisms on Cognitive Impairment in Asian Breast Cancer Patients. PLoS One . 2016;11:e0164204. doi: 10.1371/journal.pone.0164204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shah S, Ma Y, Scherzer R, Huhn G, French AL, Plankey M, Peters MG, Grunfeld C, Tien PC. Association of HIV, hepatitis C virus and liver fibrosis severity with interleukin-6 and C-reactive protein levels. AIDS . 2015;29:1325–1333. doi: 10.1097/QAD.0000000000000654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abraham BP, Ahmed T, Ali T. Inflammatory Bowel Disease: Pathophysiology and Current Therapeutic Approaches. Handb Exp Pharmacol . 2017;239:115–146. doi: 10.1007/164_2016_122. [DOI] [PubMed] [Google Scholar]
- 48.Dhingra AK, Chopra B, Dass R, Mittal SK. An update on Anti-inflammatory Compounds: A Review. Antiinflamm Antiallergy Agents Med Chem . 2015;14:81–97. doi: 10.2174/1871523014666150514102027. [DOI] [PubMed] [Google Scholar]
- 49.Jaschinski T, Mosch CG, Eikermann M, Neugebauer EA, Sauerland S. Laparoscopic vs open surgery for suspected appendicitis. Cochrane Database Syst Rev . 2018;11:CD001546. doi: 10.1002/14651858.CD001546.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ceresoli M, Tamini N, Gianotti L, Braga M, Nespoli L. Are endoscopic loop ties safe even in complicated acute appendicitis? Int J Surg . 2019;68:40–47. doi: 10.1016/j.ijsu.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 51.Myers E, Kavanagh DO, Ghous H, Evoy D, McDermott EW. The impact of evolving management strategies on negative appendicectomy rate. Colorectal Dis . 2010;12:817–821. doi: 10.1111/j.1463-1318.2009.01910.x. [DOI] [PubMed] [Google Scholar]