Figure 1.
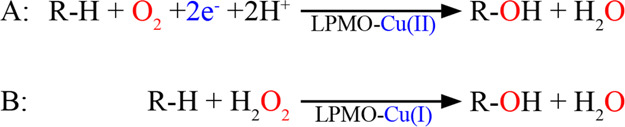
Reaction schemes for monooxygenase (A) and peroxygenase (B) reaction. The substrate is indicated by R. Hydroxylation of one of the carbons destabilizes the glycosidic bond, which, once oxidized, undergoes an elimination reaction leading to bond breakage.12 Note the potential difference in reductant consumption between the two reaction schemes. In the peroxygenase scheme, a once reduced LPMO can carry out multiple reactions,20,36,37 meaning that reductant consumption will be low if H2O2 is provided externally. If, however, H2O2 is generated in situ through the reduction of O2, also the peroxygenase reaction will require two electrons per cycle (O2 + 2e– + 2H+ → H2O2).
