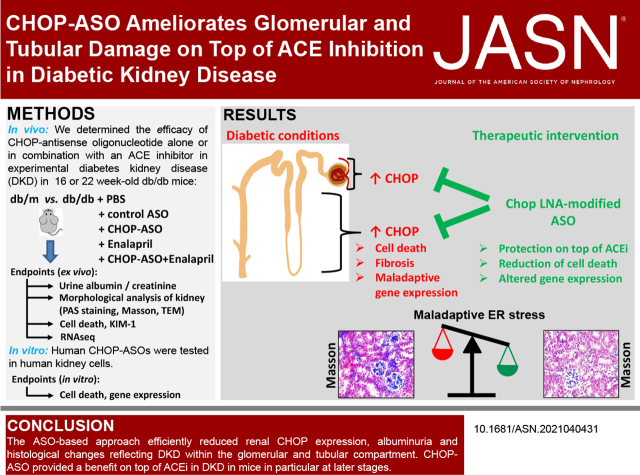
Significance Statement
The endoplasmic reticulum (ER) stress response and the maladaptive and cell-death–promoting transcription factor C/EBP homologous protein (CHOP) have been linked with diabetic kidney disease (DKD). Specific therapies targeting maladaptive ER stress signaling are lacking. We show that an antisense oligonucleotide (ASO)–based approach reducing CHOP expression ameliorates DKD in mice, providing renal protection on top of ACE inhibition. CHOP inhibition improves both glomerular and tubular damage. ASO-based therapies are a potentially new approach to target maladaptive ER stress signaling and improve DKD.
Keywords: chronic kidney disease, chronic nephropathy, diabetic nephropathy
Visual Abstract
Abstract
Background
Maladaptive endoplasmic reticulum stress signaling in diabetic kidney disease (DKD) is linked to increased glomerular and tubular expression of the cell-death–promoting transcription factor C/EBP homologous protein (CHOP). Here, we determined whether locked nucleic acid (LNA)–modified antisense oligonucleotides (ASOs) targeting CHOP ameliorate experimental DKD.
Methods
We determined the efficacy of CHOP-ASO in the early and late stages of experimental DKD (in 8- or 16-week-old db/db mice, respectively) alone or with an angiotensin-converting enzyme inhibitor (ACEi), after an in vivo dose-escalation study. We used renal functional parameters and morphologic analyses to assess the effect of CHOP-ASO and renal gene-expression profiling to identify differentially regulated genes and pathways. Several human CHOP-ASOs were tested in hyperglycemia-exposed human kidney cells.
Results
CHOP-ASOs efficiently reduced renal CHOP expression in diabetic mice and reduced markers of DKD at the early and late stages. Early combined intervention (CHOP-ASO and ACEi) efficiently prevented interstitial damage. At the later timepoint, the combined treatment reduced indices of both glomerular and tubular damage more efficiently than either intervention alone. CHOP-ASO affected a significantly larger number of genes and disease pathways, including reduced sodium-glucose transport protein 2 (Slc5a2) and PROM1 (CD133). Human CHOP-ASOs efficiently reduced glucose-induced CHOP and prevented death of human kidney cells in vitro.
Conclusions
The ASO-based approach efficiently reduced renal CHOP expression in a diabetic mouse model, providing an additional benefit to an ACEi, particularly at later timepoints. These studies demonstrate that ASO-based therapies efficiently reduce maladaptive CHOP expression and ameliorate experimental DKD.
Considering the increasing worldwide prevalence of type 2 diabetes, the incidence of diabetic kidney disease (DKD), the most common cause of terminal kidney failure, is expected to increase in future years.1 Despite promising therapeutic developments over the last decades, DKD still lacks an efficient therapy that halts or reverses the disease.2 In addition to optimized blood glucose control, inhibition of the renin–angiotensin aldosterone system is the mainstay of therapy. However, these therapies delay, but do not stop, disease progression.2 SGLT2 inhibitors have emerged as promising drugs, showing renoprotective effects in clinical studies.3 However, their long-term efficacy and safety profile remain to be established.3 Hence, there remains an unmet need for new therapies to treat DKD.
The endoplasmic reticulum (ER) stress response constitutes a cellular response closely linked with metabolic diseases, such as diabetes mellitus and DKD.4,5 Physiologically, the ER folds proteins and manages Ca2+ homeostasis. On cellular stress, the ER launches a signaling response aiming to restore protein homeostasis and thus cellular function (adaptive ER stress response). To achieve this, three signaling arms may be engaged: PERK (PKR-like ER kinase)-eIF2α (eukaryotic translation initiation factor 2 alpha), ATF6 (activating transcription factor 6), and IRE1α. The PERK-eIF2α signaling arm attenuates general protein translation, thus alleviating the protein folding load, and induces cell cycle arrest. The transcription factor ATF6 is released from the ER, and after maturation (cleavage) in the Golgi, it translocates to the nucleus to regulate gene expression.4,5 IRE1α (Inositol-requiring transmembrane kinase/endoribonuclease 1 alpha) splices XBP-1 (X-Box binding protein 1) RNA via its ribonuclease activity, promoting the expression of the primarily adaptive transcription factor XBP1 splice variant. Although the ER stress response primarily aims to restore cellular function, it will induce cell death in patients with persistent stress, largely through the transcription factor CHOP (maladaptive response).
The age-dependent induction of renal CHOP expression is accelerated in diabetic mice.6 CHOP induction is observed in murine models of diabetes and in humans with diabetes in the tubular and glomerular compartment.5,7–9 The concomitant induction of the maladaptive transcription factor CHOP in both the glomerular and tubular compartments in DKD makes CHOP an attractive therapeutic target. Indeed, nonspecific approaches to restore ER function using chaperones, such as bile-acid derivates (tauroursodeoxycholic acid, TUDCA; ursodeoxycholic acid) or 4-phenylbutyrate, demonstrated beneficial effects in experimental DKD.9,10 However, nonspecific ER chaperones may have additional effects, as demonstrated with TUDCA, which regulates FXR (farnesoid X receptor)-dependent gene expression in the tubular compartment in addition to restoring ER function.9 Whether specific targeting of the maladaptive ER stress response by inhibiting CHOP conveys beneficial effects in DKD remains unknown.
Antisense oligonucleotides (ASOs) targeting specific genes have emerged as a powerful therapeutic for a number of diseases.11–13 Because ASOs reduce, but do not completely block, the expression of targeted genes, their effect is expected to differ from those seen in knockout mice. The partially sustained expression of genes targeted by ASOs may be advantageous, because a complete loss of function typically will not restore the physiologic situation. In this study, we tested ASOs targeting CHOP expression as a potential new approach for treating DKD. We used third-generation ASOs designed as gapmers with locked nucleic acid–modified nucleotides in the flanks, which increase the affinity of the ASO to the target RNA. In contrast to earlier generations, third-generation ASOs do not require transfection reagents or conjugations for efficient target knockdown in vitro.14,15 Furthermore, specific target knockdown can be achieved in vivo without the use of complex formulations.14 The efficacy of CHOP-ASO was determined alone and in combination with an ACE inhibitor in diabetic mice at early and late stages of DKD to investigate disease prevention or therapeutic intervention, respectively.
Materials and Methods
For details, please see Supplemental Materials and Methods, the Major Resource Table, and the Supplemental References.
Mice
db/db (Lepr db/db) and nondiabetic db/m (Lepr db/+) mice (8- or 16-weeks-old) were obtained from the Jackson Laboratory (Bar Harbor, ME, USA). Only male mice were used throughout this study in accordance with the approved procedures. All animal experiments were conducted according to standards and procedures approved by the local Animal Care and Use Committee (Landesverwaltungsamt Halle, Germany).
In Vivo Intervention Studies
Male db/db mice were randomly assigned to five experimental groups at 8 weeks (disease prevention model) or 16 weeks (disease intervention model) of age as follows: control (untreated); enalapril (E) treatment alone (ACE inhibitor, 50 mg/L administered orally via drinking water)9; CHOP-ASO alone (ChA,1 mg/kg, or 3 mg/kg on alternative days, administered intraperitoneally); nonspecific control oligonucleotide (CON, 1 mg/kg on alternative days, administered intraperitoneally); and combined ACEi (E) and CHOP-ASO (ChA) treatment. A subset of db/db control mice received PBS as a vehicle control. Additionally, db/m (nondiabetic) mice were used as nondiabetic controls. After 8 (disease prevention model) or 6 (disease reversal model) weeks of treatment, mice were euthanized at 16 or 22 weeks of age, respectively, and perfused with ice-cold PBS, followed by 4% paraformaldehyde buffered in PBS. Kidneys were prepared as previously described.5,9,16,17
Determination of Albuminuria
The day before tissue preparation, mice were individually placed in metabolic cages, and urine samples were collected over a 12-hour period. Urine albumin was determined using an ELISA for mouse albumin according to the manufacturer’s instructions, and urine creatinine was determined using a commercially available assay of a modified version of the Jaffe method (X-Pand automated platform; Siemens).5,9,16,17
Statistical Analysis
Statistical analyses were performed with ANOVA and post hoc comparison with the Bonferroni method. The Kolmogorov-Smirnov test or D’Agostino–Pearson normality test was used to determine whether the data were normally distributed. StatistiXL (www.statistixl.com), Prism 5 (www.graphpad.com) and R software18 were used for statistical analyses. To assess the different distributions shown in the Venn diagram, a chi-squared test was applied. Statistical significance was accepted at values of P<0.05.
Results
Inhibition of CHOP Prevents DKD in Mice as Efficiently as an ACEi
To evaluate the role of CHOP inhibition in DKD, we first conducted a dose-escalation study (1 and 3 mg/kg, on alternate days, for 8 weeks) of CHOP-ASO (ChA) using in db/db mice, in which CHOP levels have been reported to be increased.5,6,9 Compared with PBS-treated db/db mice or mice administered CON, CHOP-ASO (ChA)–treated mice showed efficient reductions in the mRNA and protein levels of CHOP at 16 weeks (Supplemental Figure 1, A and B). No effect on body weight or blood glucose levels was observed at any tested dose (Supplemental Figure 1, C and D).
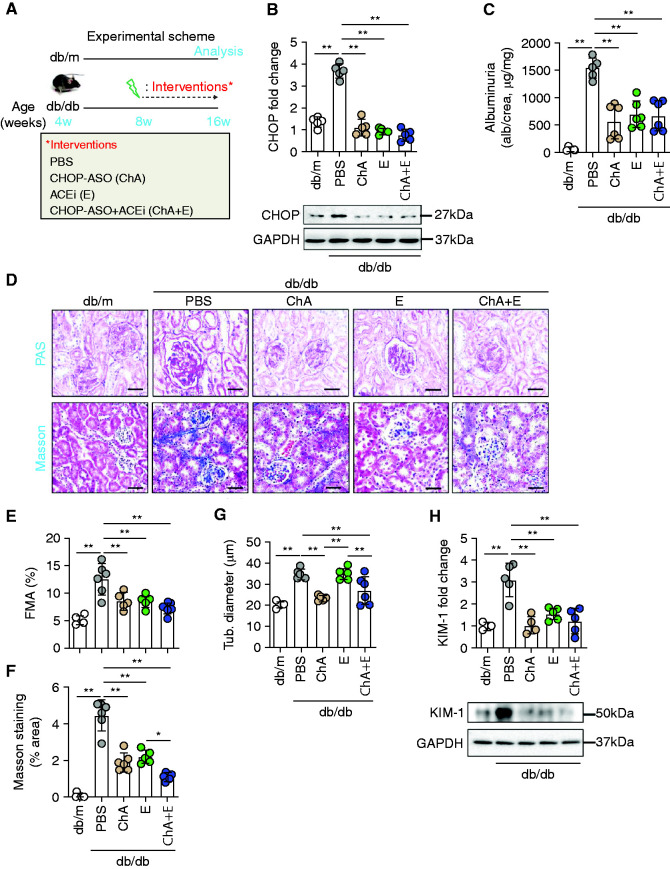
Using the lowest tested dose (1 mg/kg, on alternative days), we started a new study in db/db mice beginning at the age of 8 weeks (i.e., before the establishment of albuminuria).17 Because the PBS and CON mice did not differ in the dose-finding study (Supplemental Figure 1 and data not shown), we only included a PBS group as control. We compared the efficacy of CHOP-ASO (ChA) to that of the PBS-injected controls, the ACE inhibitor enalapril alone (ACEi, E)9 or a combination of CHOP-ASO plus ACEi (ChA+E, Figure 1A). The pharmacological interventions efficiently reduced renal CHOP expression in db/db mice (age 16 weeks), compared with the expression after treatment with PBS (Figure 1B), but had no effect on blood glucose levels or body weight (Supplemental Figure 1, E and F). In parallel, we observed a marked reduction in albuminuria (reflected by the urine albumin to creatinine ratio, Figure 1C) and improvement in glomerular injury, as reflected by mesangial matrix accumulation (fractional mesangial area; Figure 1, D and E). Likewise, both the ACEi (E) and CHOP-ASO (ChA) reduced interstitial fibrosis and the expression of the kidney injury marker KIM-1, indicating tubular protection (Figure 1, D and F–H). With regard to KIM-1 expression, no additive effect of the combined ACEi (E) and CHOP-ASO (ChA) intervention was observed, the combined intervention reduced interstitial fibrosis slightly more efficiently than either therapy alone (Figure 1, D and F), suggesting an added value of the combination therapy. Indeed, although ACEi (E) alone had no effect on tubular diameter (in agreement with previous findings9), this indicator of tubular damage was reduced by CHOP-ASO (ChA) alone or CHOP-ASO+ACEi (ChA+E, Figure 1, D and G). These data demonstrate that therapeutic inhibition of CHOP ameliorates DKD.
Figure 1.
CHOP-ASO (ChA) protects both glomerular and tubular compartments in db/db mice. (A) Experimental scheme. (B) Scatter plot with bars summarizing renal expression of CHOP (B, bottom: representative immunoblots, GAPDH was used for normalization). (C) Scatter plot with bars summarizing albuminuria levels (C, urine albumin-creatinine ratio). (D–H) Representative renal histology sections (D, periodic acid–Schiff [PAS] stain, upper panel, and Masson trichrome stain, lower panel) showing glomerular and tubular morphology. Scatter plot with bars summarizing glomerular extracellular matrix accumulation as indicated with PAS staining (E, fractional mesangial area, FMA). Scatter plot with bars summarizing renal collagen deposition using Masson’s trichrome stain (F, percentage of stained area) and tubular diameter (G, Tub. diameter). Scatter plot with bars summarizing renal expression of KIM-1 (H, top; bottom: representative immunoblots, GAPDH was used for normalization). Nondiabetic mice (db/m, 16 weeks old) and 16-week-old diabetic db/db mice treated for 8 weeks (starting at the age of 8 weeks) with PBS, ChA, E, or ChA+E. Scale bar, 50 µm (D); *P≤0.05, **P≤0.01, (B, C, and E–H: ANOVA); each dot in scatter plots represents one sample.
Combined ACE and CHOP Inhibition Halts the Progression of DKD
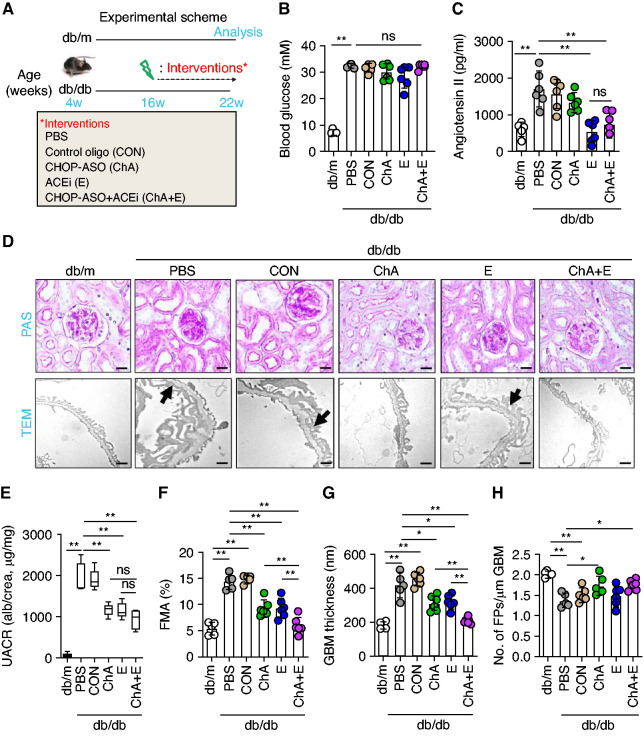
We and others have previously shown that ACEi (E) in rodents are less efficient when administered after established albuminuria.9,19 Given the efficient prevention of DKD by CHOP-ASO (ChA), we next asked whether CHOP inhibition provides an additive effect on top of ACEi (E) in rodents with established albuminuria. We used the same interventions as above in db/db mice, but started the interventions in mice aged 16 weeks (i.e., mice with established albuminuria and renal damage, Figure 2A).17 None of the interventions had an effect on blood glucose values, body weight, or parameters of liver function (plasma alanine aminotransferase, or aspartate aminotransferase, Figure 2B and Supplemental Figure 2, A–C). As expected, ACEi (E) normalized plasma levels of angiotensin II and systolic BP in db/db mice, whereas CHOP-ASO (ChA) treatment had no effect on the plasma levels of angiotensin II or systolic BP in db/db mice (Figure 2C and Supplemental Figure 2D).
Figure 2.
CHOP inhibition provides protection on top of ACEi to halt the progression of DKD. (A) Experimental scheme. (B and C) Scatter plot with bars and box plots summarizing (B) blood glucose levels and (C) plasma angiontensin II levels, respectively. (D–H) Representative renal histologic sections showing (D, upper panel) PAS staining, (D, lower panel, the arrows indicate exemplary podocyte foot processes effacement) transmission electron microscopy (TEM). (E) Box plots summarizing urine albumin-creatinine ratio (UACR). Scatter plot with bars summarizing glomerular extracellular matrix accumulation as indicated with (F) PAS staining (fractional mesangial area, FMA), (G) glomerular basement membrane thickness (GBM), and (H) number of podocyte foot processes per micrometer GBM as analyzed by TEM images. Nondiabetic mice (db/m, 22 weeks old) and 22-week-old diabetic db/db mice treated for 6 weeks (starting at the age of 16 weeks) with PBS, CON, ChA, E, or ChA+E. Scale bar, (D, upper panel) 50 µm or (D, lower panel) 1 µm; *P≤0.05, **P≤0.01, ns, nonsignificant (B, C, E–H: ANOVA); each dot in scatter plots represents one sample.
Compared with PBS-treated db/db mice or mice administered a nonspecific CON, CHOP-ASO (ChA), and ACEi (E) each reduced mesangial matrix accumulation and the combined intervention of CHOP-ASO plus ACEi (ChA+E) conveyed an added effect (Figure 2, D and F). Likewise CHOP-ASO (ChA) and ACEi (E) markedly reduced albuminuria (Figure 2E) and the combined intervention of CHOP-ASO plus ACEi (ChA+E) was slightly, although not significantly, better than either intervention alone (P=0.09 compared with ChA alone and P=0.08 compared with ACEi alone, Figure 2E). The combined intervention of CHOP-ASO plus ACEi (ChA+E) was superior with regard to reducing the glomerular basement membrane thickness (Figure 2, D and G). Of note, only CHOP-ASO (ChA), either alone or in combination with ACEi (E), partially restored the density of podocyte foot processes (number of foot processes per µm, Figure 2, D and H).
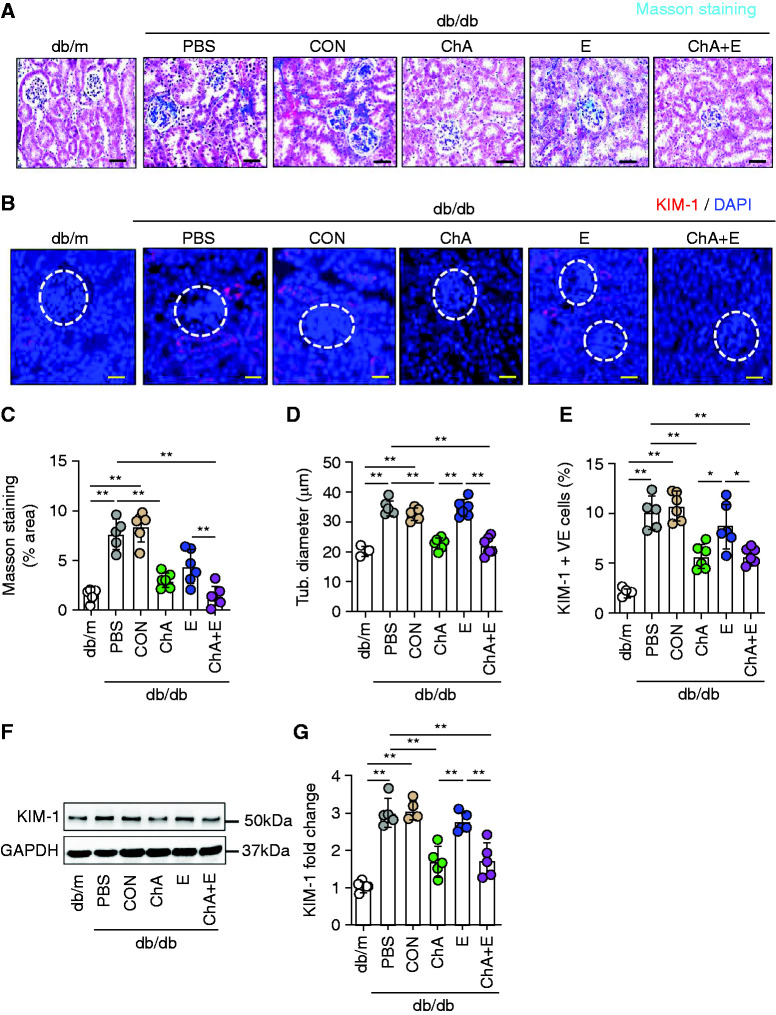
An additive effect of CHOP-ASO (ChA) and ACEi (E) was evident in the tubular compartment. Interstitial fibrosis was reduced by both CHOP-ASO (ChA) and ACEi (E) and the combination of both was slightly superior to either intervention alone (Figure 3, A and C). Of note, although ACEi (E) alone had no effect on the tubular diameter, CHOP-ASO (ChA) alone or in combination with ACEi (E) efficiently reduced the tubular diameter (Figure 3, D). Improved protection from tubular damage by either CHOP-ASO (ChA) alone or combined intervention with CHOP-ASO (ChA) and ACEi (E) was confirmed by reduced renal expression of KIM-1 by immunohistochemistry and immunoblotting (Figure 3, B and E–G).
Figure 3.
CHOP inhibition but not ACEi halts the progression of hyperglycemia-induced renal damage. (A) Representative renal histologic sections showing Masson trichrome staining of glomerular and tubular compartments. (B) Representative immunofluorescence images of KIM-1 (glomeruli indicated by the white dashed circles) expression. (C) Scatter plot with bars summarizing renal collagen deposition (Masson staining % area) using Masson’s trichrome stain and (D) renal tubular diameter. Scatter plot with bars summarizing the frequency of (E) KIM-1–positive renal cells using KIM-1 immunofluorescence staining. (F) Representative immunoblot images of renal KIM-1 expression (GAPDH was used for normalization). (G) Scatter plot with bars summarizing KIM-1 immunoblot data. Nondiabetic mice (db/m, 22 weeks old) and 22-week-old diabetic db/db mice treated for 6 weeks (starting at the age of 16 weeks) with PBS, nonspecific CON, ChA, E, or ChA+E. Scale bar, (A) 50 µm and (B) 25 µm; *P≤0.05, **P≤0.01 (C–G: ANOVA); each dot in scatter plots represent one sample.
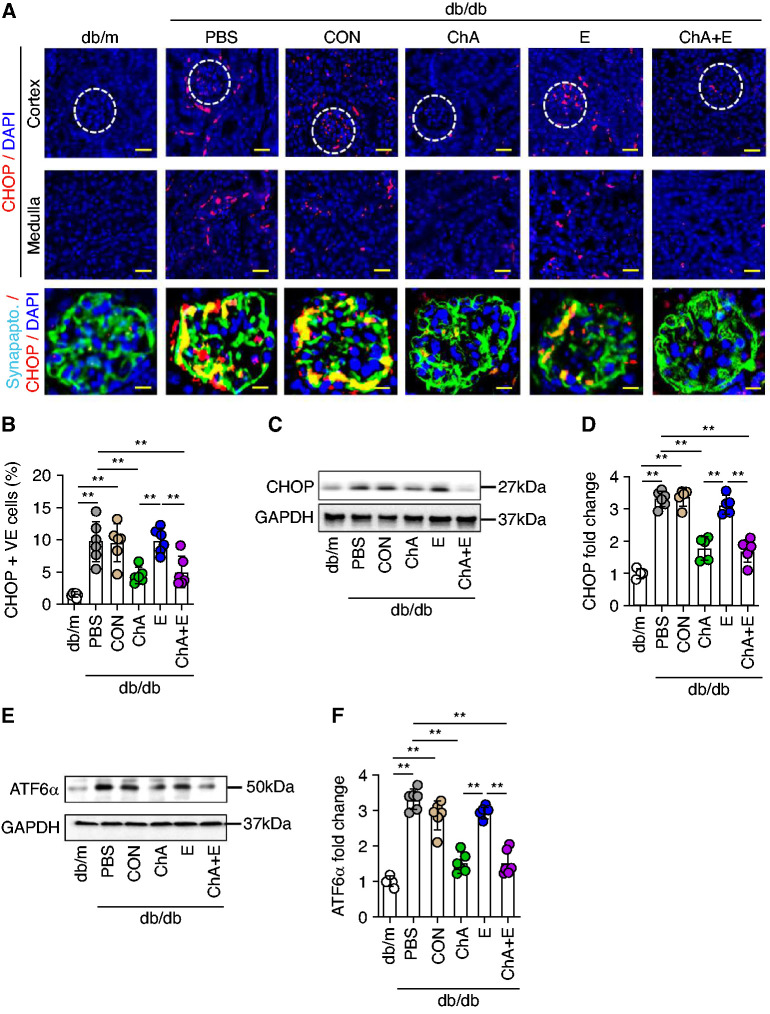
Consistent with a protective effect of CHOP-ASO (ChA) in the glomerular and tubular renal compartment, CHOP expression was induced and was associated with increased ATF6α expression in both compartments in diabetic mice (Figure 4 and Supplemental Figure 3). Both, increased CHOP and ATF6α expression were efficiently reduced by CHOP-ASO (ChA). Interestingly, unlike early intervention with ACEi (E), (Figure 1B), later intervention with ACEi (E) failed to suppress renal CHOP and ATF6α expression in glomerular and tubular compartments (immunofluorescence and immunoblotting, Figure 4 and Supplemental Figure 3), suggesting failure to suppress CHOP expression is linked to the impaired efficacy of ACEi (E) in these mice. The persistent increase of ATF6α and CHOP in ACEi (E)-treated mice supports the notion that maladaptive ER stress signaling restricts the protective effect of ACEi (E), if initiated after the establishment of albuminuria.
Figure 4.
CHOP inhibition but not ACEi reduces renal maladaptive unfolded protein response (UPR) in diabetic mice. (A) Representative immunofluorescence images of CHOP (renal cortex; top panel, glomeruli indicated by white dashed circles; renal medulla; middle panel). (A, bottom panel) Representative co-immunofluorescence images of CHOP and podocytes marker, synaptopodin. CHOP-positive cells are indicated in red, synaptopodin in green and DAPI (blue) represents nuclear counterstaining. Scatter plot with bars summarizing the frequency of CHOP -positive (B) renal cells using CHOP immunofluorescence staining. Representative immunoblot images of (C) renal CHOP expression (GAPDH was used for normalization) and (D) scatter plot with bars summarizing CHOP immunoblot data. Representative immunoblot images of (E) renal ATF6α. expression (GAPDH was used for normalization) and (F) scatter plot with bars summarizing ATF6α. immunoblot data. Nondiabetic mice (db/m, 22 weeks old) and 22-week-old diabetic db/db mice treated for 6 weeks (starting at the age of 16 weeks) with PBS, CON, ChA, E, or ChA+E. Scale bar, (A, upper and middle panels) 50 µm and (A, bottom panel) 25 µm; *P≤0.05. **P≤0.01 (B, D, and F: ANOVA); each dot in scatter plots represent one sample.
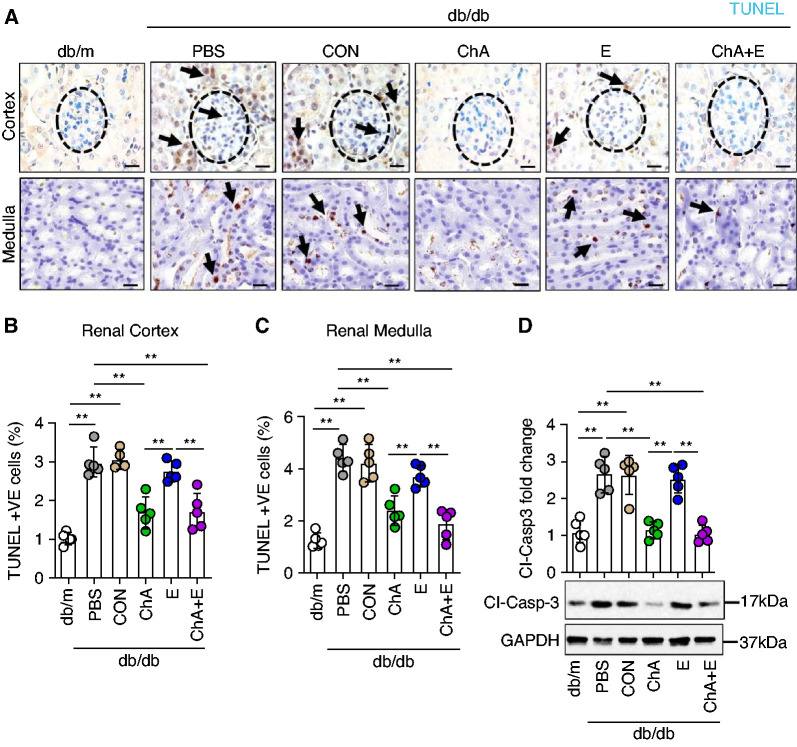
Congruent with the suppression of CHOP in both renal compartments, CHOP-ASO (ChA), either alone or in combination with ACEi (E), was superior to ACEi (E) alone in reducing the number of terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining–positive cells within the renal glomerular and the tubular compartments (Figure 5, A–C and Supplemental Figure 4). Suppression of renal cell death was confirmed by reduced levels of cleaved caspase-3 on CHOP-ASO (ChA), but not on ACEi (E) intervention (Figure 5D).
Figure 5.
CHOP inhibition but not ACEi ameliorates diabetes-induced cell death in renal glomerular and tubular compartments. (A) Representative renal histologic sections showing terminal deoxynucleotidyl transferase dUTP nick end labeling staining (TUNEL) (renal cortex, upper panel; renal medulla, bottom panel). TUNEL staining: brown, TUNEL-positive cells detected by HRP (horseradish peroxidase)-DAB (3,3'-diaminobenzidine reaction; blue, hematoxylin counterstain. The arrows show exemplary TUNEL positive cells. Scatter plot with bars summarizing the frequency of TUNEL-positive cells in (B) renal cortex and (C) medullary areas. Representative immunoblot images of renal cleaved caspase-3 (D, bottom, GAPDH was used for normalization) and (D) scatter plot with bars summarizing cleaved caspase-3 immunoblot data. Nondiabetic mice (db/m, 22 weeks old) and 22-week-old diabetic db/db mice treated for 6 weeks (starting at the age of 16 weeks) with PBS, CON, ChA, E, or ChA+E. Scale bar, 25 µm (A); *P≤0.05, **P≤0.01 (B–D: ANOVA); each dot in scatter plots represent one sample.
Inhibition of CHOP Alters Gene Expression
CHOP promotes cell death and functions as a transcriptional regulator. Considering the latter, we next performed gene expression analyses (RNA sequencing) in the cohort of mice administered treatments starting at 16 weeks. Three samples, which were clearly outliers in the principal component analyses and did not pass internal quality standards, were excluded from the analyses (one each from the PBS, the control, and the CHOP-ASO+ACEi group, ChA+E, Supplemental Figure 5A). As expected, nondiabetic db/m mice were easily distinguishable from db/db mice in the principal component analyses, irrespective of the intervention (Supplemental Figure 5B). Three further samples, two from the CHOP-ASO+ACEi (ChA+E) group and one from the ACEi (E)-only group, were excluded from further analyses due to low quality (Supplemental Figure 5B). When only comparing db/db mice, mice receiving CHOP-ASO (ChA) were clearly distinguishable from control db/db mice and db/db mice receiving CON or ACEi (E) alone (Supplemental Figure 5C).
Due to the low number of mice (n=2) within the CHOP-ASO+ACEi (ChA+E) group, and the high congruence in gene expression of this group and the CHOP-ASO (ChA) group, this group was excluded from further in silico analyses of the expression data. In db/db mice receiving CHOP-ASO (ChA), 500 genes showed similar expression to those in db/m mice (298 genes with induced expression [261+37] and 202 genes with suppressed expression [182 + 20]), reflecting genes normalized by CHOP-ASO (ChA) intervention (Supplemental Figure 6A). In comparison, 120 genes (77 induced genes [37+40] and 43 suppressed genes [20+23]) in ACEi (E)-treated mice were similar to those in db/m mice (Supplemental Figure 6A). The number of genes specifically regulated by CHOP-ASO (ChA, n=1019, 535 upregulated; 274+261 that overlap with db/m, 484 downregulated, 302+182 that overlap with db/m) was significantly larger than the number regulated specifically in response to ACEi (E, n=135, 87 upregulated [40+47], 48 downregulated [23+25]; chi-squared [1,1328]=156.58, P=1.59e-36). The larger number of genes regulated in response to CHOP-ASO (ChA) compared with ACEi (E) is congruent with CHOP being a transcriptional regulator. Importantly, the pattern of gene expression in CHOP-ASO (ChA)–treated mice reflects a change in the direction of gene expression in db/m mice (in the sense of normalized gene expression).
After correcting for multiple testing, we searched for disease-related pathways on the basis of the differentially expressed genes. Although only one gene set (Fibromyxosarcoma) showed significant enrichment in mice that were treated with ACEi (E) alone (Supplemental Table 1), 16 gene sets were identified for genes regulated by CHOP-ASO (ChA, Supplemental Table 1). The top ranked disease-related pathways regulated by CHOP-ASO (ChA) were hyperglycemia, tubulointerstitial fibrosis, hypertensive disease, malignant neoplasm of liver, and pancreatic carcinoma (Supplemental Figure 6B and Table 1). Thus, the top pathways related to CHOP-ASO (ChA)–regulated genes are also related to kidney and fibrotic diseases.
We next chose the top-ranked gene set for cluster analyses (hyperglycemia, Supplemental Figure 6C, only differentially regulated genes with an adjusted P<0.005 are shown, Supplemental Table 2). In these analyses, db/m mice were again clearly distinguishable from db/db mice, and mice receiving CHOP-ASO (ChA) alone were distinguishable from control db/db mice (PBS control or CON) and from db/db mice receiving ACEi (E, Supplemental Figure 6C). Among the top genes downregulated by CHOP-ASO (ChA) were SGLT2 (Slc5a2), the therapeutic target of the increasingly used SGLT2 inhibitors, and PROM1 (CD133), linked with tubular dedifferentiation after acute renal injury (Supplemental Figure 6C and Table 2). Suppression of SGLT2 by CHOP-ASO (ChA) was confirmed by immunoblotting and immunohistochemistry (Supplemental Figure 7). Thus, inhibiting CHOP expression conveys a strong additional effect on top of ACEi (E) with regard to renal gene expression, corroborating the functional and morphologic results shown above (Figures 2 and 3).
CHOP-ASO Suppresses Glucose-Induced Cell Damage in Human Renal Cells
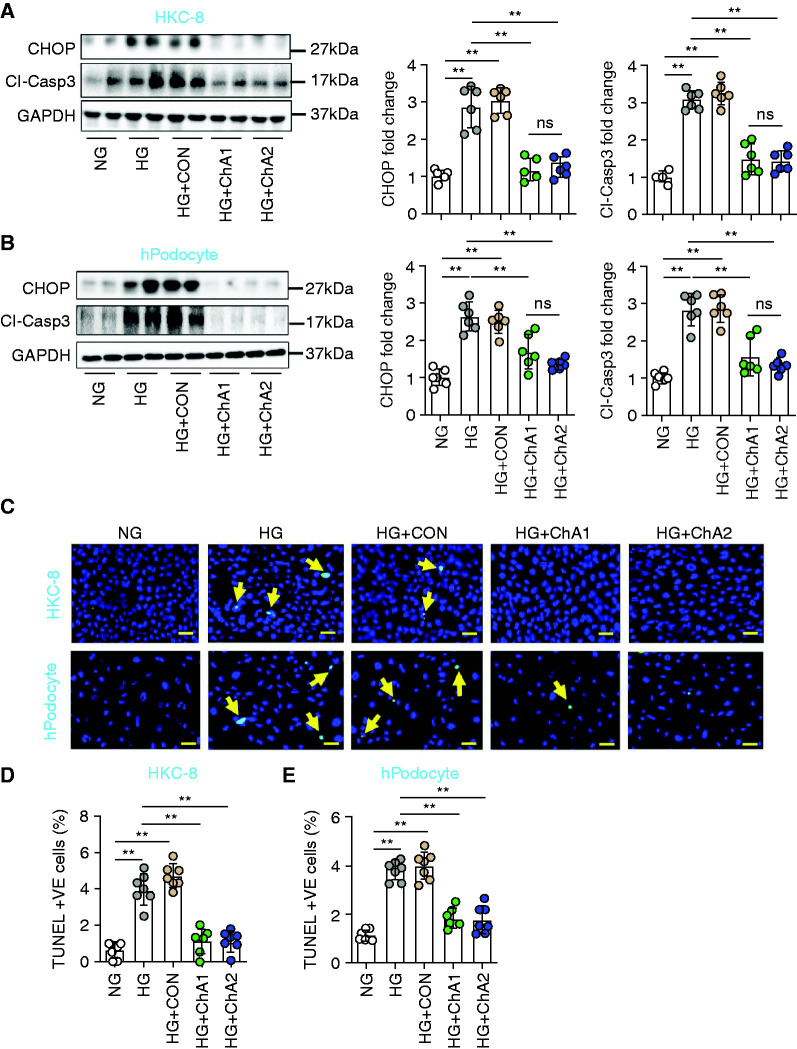
To determine whether the corresponding CHOP-ASO (ChA) convey a protective effect in human renal cells, we exposed HKC-8, podocytes, and embryonic kidney 293 cells to high glucose, resulting in increased CHOP expression (Figure 6, A and B, and Supplemental Figure 8A). Although CON had no effect on CHOP expression, two independent CHOP-ASO (ChA) efficiently reduced CHOP expression (Figure 6, A and B and Supplemental Figure 8A). Both CHOP-ASOs (ChA1 and ChA2) markedly reduced glucose-induced cell death, as identified by cleaved caspase-3 immunoblotting (Figure 6, A and B and Supplemental Figure 8A) and TUNEL staining (Figure 6, C–E and Supplemental Figure 8, B and C), whereas CON had no effect. To validate the data obtained from the unbiased analyses, we exemplarily determined the expression of renal genes regulated by CHOP-ASO (ChA). In addition to SGLT2, we analyzed PROM1 (CD133). In HKC-8 cells exposed to high glucose, we observed increased SGLT2 and PROM1 expression, which returned to normal levels on CHOP suppression with CHOP-ASO (ChA), whereas CON had no effect (Supplemental Figures 4, 6, and 9). Thus, similar to what was observed in diabetic mice, CHOP suppression reversed glucose–induced expression of selected genes and protected human renal cells from glucose-induced cellular damage.
Figure 6.
CHOP-ASO (ChA) prevents glucose-induced cell damage in human renal cells. (A and B) Representative immunoblots of CHOP and cleaved caspase-3 expression in (A, left panel) HKC-8 cells or (B, left panel, three independent repeat experiments with two technical replicates each; GAPDH was used for normalization for both cell types) human podocytes and scatter plot with bars summarizing (A and B, middle panels) the CHOP and (A and B, right panels) cleaved caspase-3 immunoblot data. (C–E) Representative immunofluorescence images of TUNEL staining of (C, upper panel) HKC-8 cells and (C, lower panel) human podocytes. TUNEL-positive cells are indicated by green fluorescence, and nuclei were counterstained with DAPI (blue). Scatter plot with bars summarizing the frequency of TUNEL-positive (D) HKC-8 cells and (E) human podocytes. Each dot in the scatter plot with bars represents averaged data from six different visual fields of individual experiments (biological replicates). NG: normal glucose (5 mM glucose plus 20 mM mannitol); HG: high glucose (25 mM); HG+CON: (25 mM glucose+10 µM nonspecific control oligonucleotide); HG+ChA1: (25 mM glucose+10 µM CHOP-ASO1); HG+ChA2: (25 mM glucose+10 µM CHOP-ASO2). Scale bar, 20 µm (C); **P≤0.01 (A, B, D and E: ANOVA). Each dot in the scatter plots represents one sample.
Discussion
Our key finding is the feasibility of targeting CHOP with CHOP-ASO (ChA), which ameliorates DKD in mice, providing an added effect on top of ACE inhibition. Although a pathogenic role of unfettered, maladaptive signaling via the ER stress response has been demonstrated in experimental and clinical studies in patients with diabetes, therapies specifically targeting the maladaptive ER stress response have not been established. In this study, we show that targeting the ER stress–dependent transcription factor CHOP, which has largely been linked with maladaptive ER stress responses, may be a suitable approach to prevent or reduce the consequences of unfettered, maladaptive ER signaling.
Previous preclinical studies using nonspecific interventions acting as ER chaperones suggested that targeting the ER stress response conveys protective effects against DKD and other diabetic complications.5,10,20,21 However, the protective effects observed using these nonspecific interventions may in part reflect “off-target” effects. TUDCA, for example, conveys additional effects via FXR-dependent signaling in the tubular compartment, and may hence be associated with both additional beneficial and adverse effects.9 This study establishes that specifically targeting the ER stress response by reducing CHOP expression is sufficient to convey protective effects in DKD. We observed protective effects of CHOP inhibition in both renal compartments (glomerular and tubular compartments) and in human renal cells (podocytes, HKC-8 cells, HEK cells). The protective effects observed in both compartments are in agreement with (1) the protective effects mediated by ER stress chaperones, and (2) the induction of CHOP in both renal compartments in DKD.5,6,9
ASOs, which are single-stranded, synthetic nucleic acids, are designed to hybridize to specific RNAs or pre-mRNAs and ultimately modulate the production of a specific protein.22 The discovery of chemical modifications that increase stability and affinity to the target RNA, such as phosphorothioated backbones and bridged nucleic acids (e.g., locked nucleic acid and constrained ethyl modifications), has allowed the development of highly potent molecules to treat an increasing number of diseases. ASOs can be administered systemically, for example, by intravenous or subcutaneous injection.23–25 By applying stringent specificity criteria during the design of ASOs, the risk of side effects caused by downregulated expression of off-target genes can be minimized and allows for the development of therapeutic ASOs with a favorable safety profile. This study is, to our knowledge, the first to show that an ASO-based therapy impairs a driver (CHOP) of maladaptive ER signaling, ameliorating DKD. Importantly, inhibiting renal CHOP expression provided a benefit on top of ACE inhibition. The synergetic effect of ACE-inhibition (E) and CHOP-ASO (ChA) does not reflect an additional lowering of BP by CHOP-ASO (ChA). We suspect the prevention of cell death and altered gene expression, such as reduced SGLT2 expression, contribute to the synergistic effects. Accordingly, ASOs specific for human CHOP reduced glucose-induced CHOP expression and cell death of human renal cells in vitro. Collectively, these data suggest inhibiting CHOP in humans to target DKD may be feasible, which needs to be elucidated in future studies.
CHOP-ASO (ChA) inhibited the expression of genes linked to renal physiology. For example, we observed an induction of CD133 in diabetic mice, which was reduced on CHOP-ASO (ChA) intervention. Because CD133 is induced on renal damage and reflects renal repair,26 we suspect its reduced expression in CHOP-ASO (ChA)–treated mice reflects attenuated renal injury. Likewise, CHOP regulated SGLT2 expression, which is known to be expressed by proximal tubular cells and to be induced in DKD.3 SGLT2 inhibitors have emerged as a new therapeutic option that not only improves the metabolic profile in patients who are diabetic, but also conveys strong renoprotective effects.3,27 Intriguingly, SGLT2 inhibition reduces ER stress in renal tubular cells.28 In this study, we show the reverse also holds true: inhibition of CHOP reduces SGLT2 expression. It appears possible but remains to be shown that CHOP and SGLT2 expression are linked by a positive regulatory mechanism that can be disrupted by inhibiting either CHOP or SGLT2 expression. Because the SGLT2 promoter does not contain a bona fide CHOP binding site, the interaction is likely indirect. Given the clinical experience, we would expect a combined intervention with SGLT2 and ACE inhibition would likewise show synergistic renoprotective effects in diabetic mice. Yet, we speculate that such combination therapy would not mimic the same changes in gene expression as observed with CHOP-ASO (ChA), considering CHOP is a transcriptional regulator, regulating in addition to SGLT2 other genes in this study. Further studies are needed to determine how CHOP regulates SGLT2 expression, to what extent the renoprotective effect of CHOP-ASO (ChA) depends on the observed reduction in SGLT2 expression, and to which extent combination therapies with ACE inhibition plus CHOP-ASO (ChA+E) or SGLT2 inhibition differ.
The efficacy of ACE inhibition varied according to the disease stage at which it was administered in this study. If administered at an early stage (8-week-old db/db mice; no albuminuria), ACE inhibition protected the mice from renal damage as efficiently as CHOP inhibition, whereas initiating ACE inhibition at a later timepoint (16-week-old db/db mice; after establishment of albuminuria) was only partially protective. This variable effect of ACE inhibition in mice is congruent with previously published data, suggesting initiation of ACE inhibition at a later time is less effective, particularly with regard to the tubular compartment.9,19 The time-dependent protective effect of ACE inhibition reflects the clinical observation that ACE inhibition is able to halt, or even reverse, newly detected microalbuminuria, although at advanced stages of DKD, ACE inhibition delays but does not halt the progression of DKD.29 Of note, protection after early (age 8 weeks) but not later (age 16 weeks) intervention with ACE inhibition was associated with reduced renal CHOP expression in this study (Figures 1B and 3, A–D), suggesting early ACE inhibition may protect against DKD, in part by inhibiting renal CHOP expression.
This study, like any, has limitations. Thus, due to restriction in the permission for in vivo mouse work, we were only able to use male mice in this study. However, we have previously observed renal CHOP induction in both sexes in diabetic mice, and hence assume CHOP-inhibition will benefit both sexes. Furthermore, in this study we used a mouse model of type 2 diabetes mellitus, but we, and others, have previously reported induction of renal CHOP expression in both type 1 and type 2 diabetic mice and humans.5,30 On the basis of the current observation and these previous studies, we speculate that CHOP-ASO (ChA)–based therapy may be a feasible therapeutic approach for both patients with type 1 and type 2 diabetes, which needs to be evaluated in future clinical studies.
As with any therapy, targeting CHOP may potentially elicit additional effects. Genetic deletion of CHOP not only ameliorates the development of DKD or the age-dependent deterioration of renal function6 (as also observed in this study) but also improves β cell survival in two independent murine diabetic models (Akita and db/db mice) and alleviates atherosclerosis in mouse models.31–33 An enhanced ER stress response is associated with obesity and has been suggested to contribute to metabolic syndrome.34 Complete CHOP deficiency has partially divergent effects on obesity and insulin sensitivity in mice: in some studies, CHOP deficiency promotes obesity, hepatic steatosis, and adipose tissue inflammation,35,36 whereas in others, CHOP deficiency protects against weight gain and maintains insulin sensitivity.37,38 In contrast, chemical chaperones convey beneficial metabolic effects,21,39 suggesting partial suppression of the unfolded protein response (UPR) stress response and CHOP with the goal of restoring ER homeostasis may convey beneficial metabolic effects. In this study, we did not observe changes in body weight, plasma alanine aminotransferase and aspartate aminotransferase levels, or blood glucose levels after interventions, suggesting that partial suppression of CHOP using an ASO-based strategy has no major metabolic effects.
Disclosures
B. Isermann reports receiving research funding from Secarna Pharmaceuticals GmbH, Munich, Germany. F. Jaschinski, R. Klar, and S. Michel are employed by Secarna Pharmaceuticals GmbH & Co. K. Shahzad reports receiving research funding from Secarna GmbH. M. Blüher reports having consultancy agreements with Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Sanofi; and reports receiving honoraria from Amgen, AstraZeneca, Bayer, Boehringer-Ingelheim, Lilly, Novartis, Novo Nordisk, Pfizer, and Sanofi. M.M. Al-Dabet reports receiving honoraria from Deutscher Akadimescher Austauchdienst. N. Klöting reports receiving research funding from DFG SFB1052 Project 209933838. P.R. Mertens reports receiving honoraria from Amgen, BMS, Boehringer Ingelheim, Genzyme, Novartis, and Roche; reports having patents and inventions including p18 protein as marker for inflammatory disease, Midkine as a marker for cardiovascular disease; and reports being a scientific advisor or member of Clinical Nephrology. All remaining authors have nothing to disclose.
Funding
This work was supported by the Deutsche Forschungsgemeinschaft (grants IS-67/8-1, 3 IS-67/11-1, CRC 1118/B07, and CRC854/B26 [to B. Isermann], SH849/1-2 and SH849/4-1 [to K. Shahzad], 4 361210922/GRK2408/P7&P9 to B. Isermann, 361210922/GRK2408/P5 to K. Shahzad, CRC854/A01, ME-1365/7-2, and ME-1365/9-2 [to P.R. Mertens]), and by Secarna Pharmaceuticals GmbH & Co. via an unrestricted grant.
Supplementary Material
Acknowledgments
M. Al-Dabet, S. Fatima, and I. Gadi performed the in vivo experiments. S. Ambreen assisted in histological analysis. A. ElWakiel and H. Khawaja assisted in ex vivo analyses. B. Isermann and K. Shahzad conceptually designed, interpreted the experimental work, and prepared the manuscript. F. Jaschinski conceptually designed the project. M. Blüher, N. Klöting, P. Mertens, and P. Nawroth provided reagents and critically discussed the data. R. Klar conceptually designed and assisted in manuscript preparation. S. Michel conducted RNA-sequencing data analysis and performed statistical analysis.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021040431/-/DCSupplemental.
Supplemental Material and Methods.
Supplemental Figure 1. Blood glucose and body weight in experimental mice.
Supplemental Figure 2. CHOP inhibition does not affect body weight, liver enzymes and systolic blood pressure in mice.
Supplemental Figure 3. CHOP inhibition but not enalapril reduces renal ATF6α levels in diabetic mice.
Supplemental Figure 4. Representative negative control for TUNEL staining of renal histologic sections.
Supplemental Figure 5. Principle component analyses and quality assessment of RNAseq analyses.
Supplemental Figure 6. CHOP Inhibition alters renal genes expression.
Supplemental Figure 7. CHOP-ASO reduces renal SGLT2 expression in diabetic mice.
Supplemental Figure 8. CHOP-ASO prevents glucose-induced cell death in HEK293 cells.
Supplemental Figure 9. CHOP-ASO prevents glucose-induced SGLT2 and PROM1 expression in HKC-8 cells.
Supplemental Table 1. Renal disease pathways targeted by CHOP inhibition in diabetic mice.
Supplemental Table 2. List of differentially expressed genes in CHOP-ASO (ChA) versus PBS treated (PBS) db/db mice in the hyperglycemia gene set.
References
- 1.Kainz A, Hronsky M, Stel VS, Jager KJ, Geroldinger A, Dunkler D, et al. : Prediction of prevalence of chronic kidney disease in diabetic patients in countries of the European Union up to 2025. Nephrol Dial Transplant 30: iv113–iv118, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Wyatt CM, Cattran DC: Intensive glycemic control and the risk of end-stage renal disease: An ADVANCE in the management of diabetes? Kidney Int 90: 8–10, 2016 [DOI] [PubMed] [Google Scholar]
- 3.Wheeler DC, James J, Patel D, Viljoen A, Ali A, Evans M, et al. ; as part of the Improving Diabetes Steering Committee : SGLT2 inhibitors: Slowing of chronic kidney disease progression in type 2 diabetes. Diabetes Ther 11: 2757–2774, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cybulsky AV: Endoplasmic reticulum stress, the unfolded protein response and autophagy in kidney diseases. Nat Rev Nephrol 13: 681–696, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Madhusudhan T, Wang H, Dong W, Ghosh S, Bock F, Thangapandi VR, et al. : Defective podocyte insulin signalling through p85-XBP1 promotes ATF6-dependent maladaptive ER-stress response in diabetic nephropathy. Nat Commun 6: 6496, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu J, Zhang R, Torreggiani M, Ting A, Xiong H, Striker GE, et al. : Induction of diabetes in aged C57B6 mice results in severe nephropathy: An association with oxidative stress, endoplasmic reticulum stress, and inflammation. Am J Pathol 176: 2163–2176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kato M, Wang M, Chen Z, Bhatt K, Oh HJ, Lanting L, et al. : An endoplasmic reticulum stress-regulated lncRNA hosting a microRNA megacluster induces early features of diabetic nephropathy. Nat Commun 7: 12864, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fan Y, Zhang J, Xiao W, Lee K, Li Z, Wen J, et al. : Rtn1a-mediated endoplasmic reticulum stress in podocyte injury and diabetic nephropathy. Sci Rep 7: 323, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marquardt A, Al-Dabet MM, Ghosh S, Kohli S, Manoharan J, ElWakiel A, et al. : Farnesoid X receptor agonism protects against diabetic tubulopathy: Potential add-on therapy for diabetic nephropathy. JASN 28: 3182–3189, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao AL, Wang L, Chen X, Wang YM, Guo HJ, Chu S, et al. : Ursodeoxycholic acid and 4-phenylbutyrate prevent endoplasmic reticulum stress-induced podocyte apoptosis in diabetic nephropathy. Lab Invest 96: 610–622, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Aghajan M, Booten SL, Althage M, Hart CE, Ericsson A, Maxvall I, et al. : Antisense oligonucleotide treatment ameliorates IFN-γ-induced proteinuria in APOL1-transgenic mice. JCI Insight 4: 126124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dias N, Stein CA: Potential roles of antisense oligonucleotides in cancer therapy. The example of Bcl-2 antisense oligonucleotides. Eur J Pharm Biopharm 54: 263–269, 2002 [DOI] [PubMed] [Google Scholar]
- 13.Rinaldi C, Wood MJA: Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat Rev Neurol 14: 9–21, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Kashyap AS, Thelemann T, Klar R, Kallert SM, Festag J, Buchi M, et al. : Antisense oligonucleotide targeting CD39 improves anti-tumor T cell immunity. J Immunother Cancer 7: 67, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stein CA, Hansen JB, Lai J, Wu S, Voskresenskiy A, Høg A, et al. : Efficient gene silencing by delivery of locked nucleic acid antisense oligonucleotides, unassisted by transfection reagents. Nucleic Acids Res 38: e3–e3, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, et al. : Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Shahzad K, Bock F, Dong W, Wang H, Kopf S, Kohli S, et al. : Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int 87: 74–84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.R Core Team (2020) : A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org/. Accessed November 2020
- 19.Lindenmeyer MT, Rastaldi MP, Ikehata M, Neusser MA, Kretzler M, Cohen CD, et al. : Proteinuria and hyperglycemia induce endoplasmic reticulum stress. JASN 19: 2225–2236, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang MK, Park SH, Kim YH, Lee EJ, Antika LD, Kim DY, et al. : Chrysin ameliorates podocyte injury and slit diaphragm protein loss via inhibition of the PERK-eIF2α-ATF-CHOP pathway in diabetic mice. Acta Pharmacol Sin 38: 1129–1140, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, et al. : Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science 313: 1137–1140, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roberts TC, Langer R, Wood MJA: Advances in oligonucleotide drug delivery. Nat Rev Drug Discov 19: 673–694, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dhuri K, Bechtold C, Quijano E, Pham H, Gupta A, Vikram A, et al. : Antisense oligonucleotides: An emerging area in drug discovery and development. JCM 9: 2004, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong D, Kurzrock R, Kim Y, Woessner R, Younes A, Nemunaitis J, et al. : AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci Transl Med 7: 314ra185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh RN, Ottesen EW, Singh NN: The first orally deliverable small molecule for the treatment of spinal muscular atrophy. Neurosci Insights 15: 2633105520973985, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corbeil D, Fargeas CA, Jászai J: CD133 might be a pan marker of epithelial cells with dedifferentiation capacity. Proc Natl Acad Sci U S A 111: E1451–E1452, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heerspink HJL, Kosiborod M, Inzucchi SE, Cherney DZI: Renoprotective effects of sodium-glucose cotransporter-2 inhibitors. Kidney Int 94: 26–39, 2018 [DOI] [PubMed] [Google Scholar]
- 28.Shibusawa R, Yamada E, Okada S, Nakajima Y, Bastie CC, Maeshima A, et al. : Dapagliflozin rescues endoplasmic reticulum stress-mediated cell death. Sci Rep 9: 9887, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ruggenenti P, Cravedi P, Remuzzi G: The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol 6: 319–330, 2010 [DOI] [PubMed] [Google Scholar]
- 30.Barati MT, Powell DW, Kechavarzi BD, Isaacs SM, Zheng S, Epstein PN, et al. : Differential expression of endoplasmic reticulum stress-response proteins in different renal tubule subtypes of OVE26 diabetic mice. Cell Stress Chaperones 21: 155–166, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oyadomari S, Koizumi A, Takeda K, Gotoh T, Akira S, Araki E, et al. : Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. J Clin Invest 109: 525–532, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thorp E, Li G, Seimon TA, Kuriakose G, Ron D, Tabas I: Reduced apoptosis and plaque necrosis in advanced atherosclerotic lesions of Apoe-/- and Ldlr-/- mice lacking CHOP. Cell Metab 9: 474–481, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou AX, Wang X, Lin CS, Han J, Yong J, Nadolski MJ, et al. : C/EBP-homologous protein (CHOP) in vascular smooth muscle cells regulates their proliferation in aortic explants and atherosclerotic lesions. Circ Res 116: 1736–1743, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hummasti S, Hotamisligil GS: Endoplasmic reticulum stress and inflammation in obesity and diabetes. Circ Res 107: 579–591, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Grant R, Nguyen KY, Ravussin A, Albarado D, Youm YH, Dixit VD: Inactivation of C/ebp homologous protein-driven immune-metabolic interactions exacerbate obesity and adipose tissue leukocytosis. J Biol Chem 289: 14045–14055, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han J, Murthy R, Wood B, Song B, Wang S, Sun B, et al. : ER stress signalling through eIF2α and CHOP, but not IRE1α, attenuates adipogenesis in mice. Diabetologia 56: 911–924, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malhi H, Kropp EM, Clavo VF, Kobrossi CR, Han J, Mauer AS, et al. : C/EBP homologous protein-induced macrophage apoptosis protects mice from steatohepatitis. J Biol Chem 288: 18624–18642, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rahman SM, Schroeder-Gloeckler JM, Janssen RC, Jiang H, Qadri I, Maclean KN, et al. : CCAAT/enhancing binding protein beta deletion in mice attenuates inflammation, endoplasmic reticulum stress, and lipid accumulation in diet-induced nonalcoholic steatohepatitis. Hepatology 45: 1108–1117, 2007 [DOI] [PubMed] [Google Scholar]
- 39.Kars M, Yang L, Gregor MF, Mohammed BS, Pietka TA, Finck BN, et al. : Tauroursodeoxycholic acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes 59: 1899–1905, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.