Abstract
The European Commission requested EFSA to assess if different thermal processes achieve a 5 log10 reduction in Enterococcus faecalis or Salmonella Senftenberg (775W) and (if relevant) a 3 log10 reduction in thermoresistant viruses (e.g. Parvovirus) as well as if different chemical processes achieve a 3 log10 reduction of eggs of Ascaris sp., in eight groups of Category 2 and 3 derived products and animal by‐products (ABP). These included (1) ash derived from incineration, co‐incineration and combustion; (2) glycerine derived from the production of biodiesel and renewable fuels; (3) other materials derived from the production of biodiesel and renewable fuels; (4) hides and skins; (5) wool and hair; (6) feathers and down; (7) pig bristles; and (8) horns, horn products, hooves and hoof products. Data on the presence of viral hazards and on thermal and chemical inactivation of the targeted indicator microorganisms and biological hazards under relevant processing conditions were extracted via extensive literature searches. The evidence was assessed via expert knowledge elicitation. The certainty that the required log10 reductions in the most resistant indicator microorganisms or biological hazards will be achieved for each of the eight groups of materials mentioned above by the thermal and/or chemical processes was (1) 99–100% for the two processes assessed; (2) 98–100% in Category 2 ABP, at least 90–99% in Category 3 ABP; (3) 90–99% in Category 2 ABP; at least 66–90% in Category 3 ABP; (4) 10–66% and 33–66%; (5) 1–33% and 10–50%; (6) 66–90%; (7) 33–66% and 50–95%; (8) 66–95%, respectively. Data generation on the occurrence and reduction of biological hazards by thermal and/or chemical methods in these materials and on the characterisation of the usage pathways of ABP as organic fertilisers/soil improvers is recommended.
Keywords: animal by‐products, fertilisers, compost, inactivation, Salmonella, Enterococcus, Ascaris, Parvovirus
Summary
Under the framework of Article 29 of Regulation (EC) No 178/2002, the European Commission requested EFSA to conduct an assessment of the biological risks to animal and public health deriving from the use as organic fertilisers and soil improvers (OF/SI) of the following Category 2 and 3 materials and derived products: biogas digestion residues and compost; ash derived from incineration, co‐incineration and combustion; glycerine and other products of materials derived from the production of biodiesel and renewable fuels; pet food; feed and dog chews; hides and skins; wool and hair; feather and downs; and pig bristles.
Following the clarification of the Terms of Reference (ToR), the European Commission requested EFSA to assess the requirements for alternative transformation parameters for biogas and composting plants in terms of the validation of the intended process, referred to in point 1, Section 2, Chapter III, Annex V of Commission Regulation (EU) No 142/2011, when applied to the following eight groups of Category 2 and Category 3 materials and derived products processed or obtained in accordance with Regulation (EC) No 1069/2009 and Commission Regulation (EU) No 142/2011 for the declaration of the end points in the manufacturing chain and the standard or alternative methods approved for this purpose: (1) ash derived from incineration, co‐incineration and combustion; (2) glycerine derived from the production of biodiesel and renewable fuels; (3) other materials derived from the production of biodiesel and renewable fuels; (4) hides and skins; (5) wool and hair; (6) feathers and down; (7) pig bristles; and (8) horns, horn products, hooves and hoof products.
Point 1 of Section 2 of Chapter III of Annex V of Commission Regulation (EU) No 142/2011 states that the validation of the intended process must demonstrate that the process achieves the following overall risk reduction: for thermal and chemical processes, a reduction of 5 log10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H2S negative), and a reduction in the infectivity titre of thermoresistant viruses such as parvovirus by at least 3 log10, whenever they are identified as a relevant hazard; and as regards chemical processes also a reduction of resistant parasites such as eggs of Ascaris sp. by at least 99.9% (3 log10) of viable stages.
The parameters of the processes were extracted from Annex III, Annex IV and Annex XIII of Commission Regulation (EU) No 142/2011. In the materials for which processing time was not clearly stated in the legislation (group 5 – wool and hair, and group 7 – pig bristles), two plausible scenarios were explored: 5 min and 60 min (covered in assessment question 1 (AQ1) (see Protocol in Annex A). An extensive literature search was conducted to identify viral hazards for humans and animals that have been isolated in the eight groups of materials included in the mandate. Hazards intrinsically present in the matrix were considered, while hazards deriving from external sources or cross‐contamination were excluded from the hazard identification (AQ3). Parvoviridae were considered, as a worse‐case scenario, when no intrinsic viral hazards were identified. Extensive literature searches were conducted to extract data from the scientific literature about thermal and/or chemical inactivation of E. faecalis, S. Senftenberg (AQ2), the selected viruses (non‐enveloped viruses) (AQ4) and eggs of Ascaris sp. (AQ5)
For each of the assessment questions (AQ), a body of evidence was built by summarising the data extracted from the literature on thermal and chemical inactivation of the indicator microorganisms and biological hazards for each of the materials and derived products listed in the ToR, and the existing uncertainties. Using this body of evidence, an expert knowledge elicitation procedure was performed involving eight experts (six Working Group members and two EFSA staff) who answered the following questions: (a) What is the probability that a 5 log10 reduction of E. faecalis is achieved in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved? (b) What is the probability that a 5 log10 reduction of S. Senftenberg (775 W, H2S negative) is achieved in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved? (c) What is the probability that a 3 log10 reduction of Parvovirus or the identified most resistant viruses is achieved in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved? (d) What is the probability that a 3 log10 reduction of eggs of Ascaris sp. is achieved in more than 99% of the cases, by application of the relevant chemical process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
To answer these questions, first, subjective probability ranges were provided by each of the individual experts for the 52 different combinations of materials and derived products, processes and indicator microorganisms and biological hazards. Second, after an open discussion, a single consensus probability range was obtained for each combination, based on the estimates provided by the individual experts. These consensus probability ranges are considered to best represent the uncertainty on whether the indicated log10 reductions are achieved by the standard processes for the different materials. The probability ranges for the most resistant indicator microorganisms and biological hazards were selected as the worst‐case scenario to describe the uncertainty around the overall efficacy of the standard or alternative transformation processes for animal by‐products (ABP) declaration of end points or placement in the market, defined by time/temperature/pH parameters.
The certainty that, at least in 99% of cases, the transformation processes as defined in the legislation, or in the scenarios agreed by the Working Group, are able to reduce the indicator microorganisms and biological hazards to the required extent, was judged to be as follows:
Ash derived from incineration, co‐incineration and combustion: 99–100% for both 850°C, > 2 s and 1,100°C, > 0.2 s (E. faecalis, S. Senftenberg and Parvoviridae);
Glycerine derived from the production of biodiesel and renewable fuels: 98–100% for Category 2 material subjected to Method 1 at 133°C, 20 min, 3 bar, followed by esterification and transesterification (E. faecalis, S. Senftenberg and Parvoviridae); 90–95% and 90–99% for Category 3 material subjected to 80°C for 120 min and 100°C for 60 min, followed by transesterification, respectively (Parvoviridae). Since method 5 must ensure that the two time–temperature combinations are met, even if they were assessed separately, for Category 3 material it is considered at least 90–99% certain that the transformation process is able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
Other materials derived from the production of biodiesel and renewable fuels: 90–99% for Category 2 material subjected to Method 1 at 133°C, 20 min, 3 bar (E. faecalis, S. Senftenberg and Parvoviridae); and 33–90% and 66–90% for Category 3 material subjected to 80°C for 120 min and 100°C for 60 min, respectively (Parvoviridae). Since method 5 must ensure that the two time–temperature combinations are met, even if they were assessed separately, for Category 3 material, it is considered at least 66–90% certain that the transformation process is able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
Hides and skins: 10–66% and 33–66% for pH 12–13 for 8 h, and pH 12 for > 8 h followed by pH < 3 for 16 h, respectively (eggs of Ascaris sp.);
Wool and hair: 1–33% and 10–50% for pH > 12–13, applied for 5 or 60 min, respectively (eggs of Ascaris sp.);
Feathers and down: 66–90% for 100°C for at least 30 min (Anelloviridae and Circoviridae);
Pig bristles: 33–66% and 50–95% for 100°C, applied for 5 or 60 min, respectively (Parvoviridae);
Horns, horn products, hooves and hoof products: 66–95% for 80°C for 60 min (E. faecalis and S. Senftenberg).
In order to reduce the uncertainty of the assessment, it is recommended to generate data on the occurrence of biological hazards, the intrinsic physico‐chemical properties (e.g. pH, water activity (aw)), and on the thermal and non‐thermal (chemical) inactivation of the indicator microorganisms in ABP matrices.
1. Introduction
1.1. Background and Terms of Reference as provided by the requestor
Derived products referred to in Article 3(2) of Regulation (EC) No 1069/20091 that have reached the end point in the manufacturing chain of animal by‐products may subsequently be placed on the market without restrictions under this Regulation and shall no longer be subject to official controls in accordance with Regulation (EC) No 1069/2009 and Regulation (EU) 2017/6252.
Article 32 of Regulation (EC) No 1069/2009 provides rules for placing on the market and use of organic fertilisers and soil improvers (OF/SI) of Category 2 and 3 materials. By the exclusion of Category 1 material from the production of OF/SI certain risks to animal and public health and to the environment are already addressed. However, for the use of those animal by‐products, which are classified as Category 2 material referred to in Article 9(c), a specific risk assessment is necessary to ensure that the use of such animal by‐products as organic fertiliser does not entail a risk to animal and public health and to the environment.
Regulation (EU) 2019/10093 establishing rules for the placing on the market of EU fertilising products, introduced in Article 5(2) of Regulation (EC) No 1069/2009 a reference to Article 32 of that Regulation, and thus the possibility to determine the end point in the manufacturing chain of OF/SI.4
Derived products of Category 2 and Category 3 materials,5 referred to respectively in Articles 9 and 10 of Regulation (EC) No 1069/2009, may be placed on the market and used as OF/SI. Those materials present comparable TSE/BSE risks since none of them includes specific risk materials as defined in Article 3(1)(g) of Regulation (EC) No 999/20016.
The European Parliament and the Council asked the Commission to initiate an assessment of derived products referred to in Article 32 that are already widely used in the Union as OF/SI.
This assessment shall cover at least the following products:
meat meal, bone meal, meat‐and-bone meal, hydrolysed proteins of Category 3 materials,
processed manure, compost, biogas digestion residues, feather meal, glycerine and other products of Category 2 or 3 materials derived from the production of biodiesel and renewable fuels,
pet food, feed and dog chews that have been refused for commercial reasons or technical failures,
derived products from blood of animals, hides and skins, hoofs and horns, guano of bats and birds, wool and hair, feather and downs, and pig bristles.
The following derived products are widely placed on the market for use as OF/SI:
-
1
Biogas digestion residues and compost referred to in the second subparagraph of Article 32(1) of Regulation (EC) No. 1069/2009. Standard transformation parameters for compost and biogas transformation residues are laid down in Section 1 of Chapter III of Annex V to Regulation (EU) No 142/20117. With reference to point 2(a) of Section 1 and point 2(b) of Section 2 of Chapter II of the aforementioned Annex V, Category 2 materials intended for compost or biogas transformation should be processed in accordance with processing method 1. Biogas and compost transformation has been subject to several EFSA assessments in 20058; 20099 and 201510
-
2
Ash derived from incineration, co‐incineration and combustion, carried out in accordance with methods laid down in Annex III to Regulation (EU) No 142/2011, of Category 2 and 3 materials and derived products may be used as fertilising products. The disposal of animal by‐products, including meat‐and-bone meal, by incineration, co‐incineration and combustion has been subject to EFSA assessment on several occasions. The following scientific opinions led to the conclusion that direct incineration of carcasses and incineration or burning under appropriate controlled condition of rendered material are economically feasible technologies for safe disposal of TSE risk material:
Overview of the BSE risk assessments of the European Commission's Scientific Steering Committee (SSC) and its TSE/BSE ad hoc Group11;
Opinion on open burning of potentially TSE‐infected animal materials adopted by the Scientific Steering Committee at its meeting of 16‐17 January 200312;
Opinion on the use of small incinerators for BSE risk reduction by the Scientific Steering Committee meeting of 16‐17 January 200313; and
Opinion of the Scientific Panel on Biological Hazards of the European Food Safety Authority on the “Quantitative risk assessment of the animal BSE risk posed by meat and bone meal with respect to the residual BSE risk”.14
Based on those scientific opinions, standards for the disposal of animal by‐products as waste by incineration, the disposal or recovery by co‐incineration and the use as a fuel for combustion have been laid down in Regulation (EU) No 142/201115.
-
3
Article 3 of Regulation (EU) No 142/2011 sets out end points for certain Category 2 and 3 materials which may be used in the manufacturing of OF/SI, such as derived products referred to in points (b) to (h) or side product of biofuel and oleochemical production referred to in points (a), (i), (j) and (k) of that Article.
Certain end points have been determined based on EFSA assessments of 2004,16 2010,17 201118 and 2015.19
1.1.1.
Terms of Reference
In the light of the above, and in accordance with Article 29 of Regulation (EC) No 178/200220, the Commission requests EFSA to provide a scientific opinion concerning the capacity of certain specific processing or transformation methods used in the production of organic fertilisers and soil improvers (OF/SI) in view of determining the endpoints in the manufacturing chain of CE‐marked EU fertilising products.
In particular, the scientific opinion should comprise an assessment of the biological risks to animal and public health deriving from the use as OF/SI of the following Category 2 and 3 materials and derived products processed in accordance with Regulation (EC) No 1069/2009 and Regulation (EU) No 142/2011:
biogas digestion residues and compost;
ash derived from incineration, co‐incineration and combustion;
glycerine and other products of materials derived from the production of biodiesel and renewable fuels;
pet food;
feed and dog chews;
hides and skins;
wool and hair;
feather and downs;
and pig bristles.
1.2. Interpretation of the Terms of Reference
As a result of discussions conducted with the requestor, on 25 January 2021, the European Commission indicated to EFSA that the Terms of Reference (ToR) are clarified as follows:
‘EFSA is requested to assess the requirements for alternative transformation parameters for biogas and composting plants in terms of the validation of the intended process, referred to in point 1 of Section 2 of Chapter III of Annex V to Regulation (EU) No 142/2011, when applied to other derived products. In particular, the scientific opinion should comprise an assessment of the following Category 2 and 3 materials and derived products processed or obtained in accordance with Regulation (EC) No 1069/2009 and Regulation (EU) No 142/2011 for the declaration of the end points in the manufacturing chain and the standard or alternative methods approved for this purpose:
ash derived from incineration, co‐incineration and combustion;
glycerine derived from the production of biodiesel and renewable fuels;
other materials derived from the production of biodiesel and renewable fuels;
hides and skins;
wool and hair;
feather and downs;
pig bristles;
horns, horn products, hoofs and hoof products.’
The requestor clarified that only the transformation processes included in Commission Regulation (EU) 142/2011 for the declaration of the end points in the manufacturing chain (for raw materials) and the approved standard or alternative methods to produce derived products should be considered, disregarding the further transformation processes, the fertiliser industry might apply to produce the final OF/SI, and without considering the use or applications of the final OF/SI product. It was also confirmed that only materials produced in the EU should be considered, as all imported materials from non‐EU countries will have to comply with the EU legislation. The requestor also provided an updated list of Category 2 and 3 animal by‐products (ABP) and derived materials that should be included in the assessment.
Based on this clarification, biogas digestion residues (digestate) and compost, pet food, feed and dog chews, initially included in the ToR, were excluded from the assessment. A new ABP group, horns, horn products, hooves and hoof products, was added.
Thus, it was requested to assess for the list of materials included in the ToR if: (i) the transformation processes for the declaration of the end points in the manufacturing chain or (ii) the standard or alternative methods for the production of ABP derived products meet the requirements for alternative transformation parameters for biogas and composting plants in terms of the validation of the intended process, referred to in point 1 of Section 2 of Chapter III of Annex V to Regulation (EU) No 142/2011.
It is important to emphasise that, as a result of the new request from the European Commission, the output of the scientific opinion was not a full risk assessment, but consisted of the estimation of the level of inactivation/reduction of concentration of biological hazards and indicator microorganisms of interest after the processing methods and standard or alternative methods are applied. Thus, the output of the assessment did not conclude on any relationship between the presence of hazards and the risks to human or animal health of the OF/SI containing them. Moreover, the output of this scientific opinion comprises an evaluation of certain processes applied to produce or transform a list of materials that can be further processed, in the form of composting or any other method, and used as an OF/SI.
As some of the materials in the list of the ToR include multiple substrates/matrices, it was agreed to use throughout the opinion the term ‘group’ followed by a number in the order of the ToR for each of the eight items included in it, as follows: group 1: ash derived from incineration, co‐incineration and combustion; group 2: glycerine derived from the production of biodiesel and renewable fuels; group 3: other materials derived from the production of biodiesel and renewable fuels; group 4: hides and skins; group 5: wool and hair; group 6: feathers and down; group 7: pig bristles; and group 8: horns, horn products, hooves and hoof products.
The materials in the groups included in the mandate are of two very different natures: some of them are residues or derived materials produced during the treatment of raw ABP with approved standard or alternative methods (Groups 1, 2, 3); and others are raw ABP (Groups 4, 5, 6, 7 and 8). In the former, the parameters of the standard or alternative methods will be applied, whereas in the latter, the parameters of the treatments required for the declaration of end points (Groups 4, 5 and 6) or the treatments for movement of the material between regions (Group 7) or for the placing on the market (Group 8) will be assessed. These differences will have an impact on the hazard identification conducted for each type of materials (see Section 3).
1.2.1. Background legislation and approach to answer the ToR
Commission Regulation (EU) No 142/2011, in Section 1, Chapter III, Annex V, details the minimum requirements of Category 3 ABP to be used as raw materials in a biogas or compost plant, as follows:
maximum particle size before entering the unit or the composting reactor: 12 mm;
minimum temperature in all material in the unit or in the reactor: 70°C; and
minimum time in the unit without interruption: 60 min.
Section 2.1 of the same Chapter describes the alternative transformation parameters for biogas and composting plants. According to it, the competent authority may authorise the use of parameters other than the parameters set out in point 1 of Section 1 of Chapter I and other than the standard transformation parameters, provided that the method is demonstrated to be at least as safe as the standard method. That demonstration shall include a validation, which shall be carried out in accordance with the following requirements:
-
a
Identification and analysis of possible hazards, including the impact of input material, based on a full description of the transformation conditions and parameters;
-
b
A risk assessment, which evaluates how the specific transformation conditions referred to in point (a) are achieved in practice under normal and atypical situations;
-
c
Validation of the intended process by measuring the reduction of viability/infectivity of:
-
endogenous indicator organisms during the process, where the indicator is:
-
—
consistently present in the raw material in high numbers,
-
—
not less heat resistant to the lethal aspects of the transformation process, but also not significantly more resistant than the pathogens for which it is being used to monitor,
-
—
relatively easy to quantify and to identify and to confirm; or
-
—
a well‐characterised test organism or virus, during exposure, introduced in a suitable test body into the starting material.
-
d
The validation of the intended process referred to in point (c) must demonstrate that the process achieves the following overall risk reduction:
-
i
for thermal and chemical processes by:
-
—
a reduction of 5 log 10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H 2 S negative),
-
—
reduction of infectivity titre of thermoresistant viruses such as parvovirus by at least 3 log 10 , whenever they are identified as a relevant hazard; and
-
—
-
ii
as regards chemical processes also by:
-
—
a reduction of resistant parasites such as eggs of Ascaris sp. by at least 99,9% (3 log 10 ) of viable stages;
-
—
-
e)
Designing a complete control programme including procedures for monitoring the functioning of the process referred to in point (c);
-
f)
Measures ensuring continuous monitoring and supervision of the relevant process parameters fixed in the control programme when operating the plant.
-
i
Details on the relevant process parameters used in a biogas or composting plant as well as other critical control points must be recorded and maintained so that the owner, operator or their representative and the competent authority can monitor the operation of the plant.
Records must be made available by the operator to the competent authority on request. Information relating to a process authorised under this point must be made available to the Commission on request.
Taking into account the content of this EU Regulation, an assessment was undertaken to determine if the processing standards for the declaration of the end points in the manufacturing chain or the standard or alternative methods approved for the production of derived products from the ABP in the list of materials mentioned in Section 1.2 achieve: (i) a reduction of 5 log10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H2S negative) and a reduction of infectivity titre by at least 3 log10 of those thermoresistant viruses that are identified as a relevant hazard, and, in the case of chemical processes, also a reduction of eggs of Ascaris sp. by 3 log10. This was achieved by addressing the following assessment questions (AQ):
AQ1: What are the technical parameters of the transformation processes for the declaration of the end points in the manufacturing chain, and the standard or alternative methods approved to produce derived materials or residues as described in the legislation (Commission Regulation (EU) 142/2011) of the Category 2 and 3 materials and derived products as defined in the mandate?
AQ2: Is the 5 log10 reduction of the indicator microorganisms Enterococcus faecalis (EF) or Salmonella Senftenberg (SS) achieved for each of the Category 2 and 3 materials and derived products, as defined in the clarification of the mandate, by the technical parameters identified in AQ1?
AQ3: Which viral hazards can be intrinsically found in the Category 2 and 3 materials and derived products as defined in the clarification of the mandate?
AQ4: Is the 3 log10 reduction of the selected thermoresistant viruses identified in AQ3 achieved for each of the Category 2 and 3 materials and derived products, as defined in the clarification of the mandate, by the technical parameters identified in AQ1?
AQ5: Is the 3 log10 reduction of eggs of Ascaris sp. achieved for each of the Category 2 and 3 materials and derived products, as defined in the clarification of the mandate, by the technical parameters identified in AQ1 for the processes based on chemical treatments (group 4 – hides and skins; group 5 – wool and hair)?
2. Data and methodologies
2.1. Parameters of the transformation processes (AQ1)
Two methods were applied to ascertain technical parameters of the transformation processes for the declaration of the end points in the manufacturing chain, and the standard or alternative methods as described in the legislation for the groups of materials defined in the mandate:
For materials for which details of the technical parameters of the transformation processes are explicit in the legislation: Annexes III, IV and XIII of Commission Regulation (EU) No 142/2011 were reviewed.
For materials for which details of the technical parameters of the transformation processes are not explicit in the legislation: two plausible scenarios (5 min and 60 min) were assessed.
In cases where the technical parameters were explicitly defined in the legislation, they were used as reference parameters for the assessment. When they were not explicitly defined, the uncertainty associated with the interpretation of the legislation was taken into account and described. The selected technical parameters are presented in Section 3 and applied in the expert knowledge elicitation (EKE) by producing 52 combinations of processes, materials and hazards.
2.2. Viral hazards identification (AQ3)
To identify viral hazards for humans and animals that can be found intrinsically in the Category 2 and 3 materials and derived products as defined in the list of materials provided in the clarification of the mandate, an extensive literature search was conducted. The selection of studies was based on experimental or observational studies in which the viral species, genus or family are mentioned in relation to the materials included in the mandate (raw materials). The database used was Scopus, Elsevier (English language, worldwide and not restricted in years). The search strings were designed by combining the biological hazards (virus), the matrix (material as in the mandate) and ABP. The latter group of terms was added to reduce the number of false‐positive hits. The search strings are displayed in Table A.2 in Annex A. A table with the identified viral hazards was produced with the following fields: material (Category 2 and 3 material or derived product), characteristics (non‐enveloped, DNA/RNA), family of the viral hazard, viral hazard, (main) species affected and reference/s (see Table 4 in Section 3.5).
Table A.2.
Details of the data points used to produce Figure 5 from the references identified in the literature review for Salmonella Senftenberg
| Hazard | Product group | Product/or medium | Treatment | pH | T (°C) | D (min) | 5D (min) | Ref |
|---|---|---|---|---|---|---|---|---|
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid whole eggs | Heat | 55 | 34.3 | 171.5 | Doyle and Mazzotta (2000) | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid whole eggs | Heat | 60 | 5.6 | 28 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid whole eggs | Heat | 64 | 2.8 | 14 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg yolks | Heat | 55 | 42 | 210 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg yolks | Heat | 60 | 11.8 | 59 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 55 | 3 | 15 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 60 | 0.8 | 4 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Raw milk | Heat | 60 | 0.122 | 0.61 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Raw milk | Heat | 61.5 | 0.107 | 0.535 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Raw milk | Heat | 63 | 0.067 | 0.335 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Raw milk | Heat | 64.5 | 0.067 | 0.335 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Raw milk | Heat | 67.5 | 0.046 | 0.23 | ||
| Salmonella ser. Senftenberg | Solid food product | Ground beef | Heat | 53 | 53 | 265 | ||
| Salmonella ser. Senftenberg | Solid food product | Ground beef | Heat | 58 | 15.2 | 76 | ||
| Salmonella ser. Senftenberg | Solid food product | Ground beef | Heat | 63 | 2.08 | 10.4 | ||
| Salmonella ser. Senftenberg | Solid food product | Ground beef | Heat | 68 | 0.22 | 1.1 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | PO4 | Heat | 55 | 13 | 65 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | PO4 | Heat | 65 | 0.29 | 1.45 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | PO4 | Heat | 54.4 | 14.23 | 71.15 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | PO4 | Heat | 57.2 | 6.23 | 31.15 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | PO4 | Heat | 60 | 2.69 | 13.45 | ||
| Salmonella ser. Senftenberg S2 | Liquid culture medium | PO4 | Heat | 54.4 | 17.13 | 85.65 | ||
| Salmonella ser. Senftenberg S2 | Liquid culture medium | PO4 | Heat | 57.2 | 7.14 | 35.7 | ||
| Salmonella ser. Senftenberg S2 | Liquid culture medium | PO4 | Heat | 60 | 2.88 | 14.4 | ||
| Salmonella ser. Senftenberg R1 | Liquid culture medium | PO4 | Heat | 54.4 | 19.32 | 96.6 | ||
| Salmonella ser. Senftenberg R1 | Liquid culture medium | PO4 | Heat | 57.2 | 3.72 | 18.6 | ||
| Salmonella ser. Senftenberg R1 | Liquid culture medium | PO4 | Heat | 60 | 3.06 | 15.3 | ||
| Salmonella ser. Senftenberg R2 | Liquid culture medium | PO4 | Heat | 54.4 | 12.77 | 63.85 | ||
| Salmonella ser. Senftenberg R2 | Liquid culture medium | PO4 | Heat | 57.2 | 5.39 | 26.95 | ||
| Salmonella ser. Senftenberg R2 | Liquid culture medium | PO4 | Heat | 60 | 2.31 | 11.55 | ||
| Salmonella ser. Senftenberg R6 | Liquid culture medium | PO4 | Heat | 54.4 | 13.14 | 65.7 | ||
| Salmonella ser. Senftenberg R6 | Liquid culture medium | PO4 | Heat | 57.2 | 5.56 | 27.8 | ||
| Salmonella ser. Senftenberg R6 | Liquid culture medium | PO4 | Heat | 60 | 1.92 | 9.6 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 50 | 268 | 1340 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 55 | 36.2 | 181 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 60 | 6.3 | 31.5 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 50 | 146 | 730 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 55 | 4.9 | 24.5 | ||
| Salmonella ser. Senftenberg | Liquid culture medium | HI | Heat | 60 | 0.62 | 3.1 | ||
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9 | 52.2 | 28.6 | 143 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9 | 55 | 7.2 | 36 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9 | 56.7 | 3.1 | 15.5 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9 | 52.2 | 3.1 | 15.5 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9 | 55 | 0.78 | 3.9 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9.5 | 52.2 | 19.3 | 96.5 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9.5 | 55 | 4.9 | 24.5 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9.5 | 56.7 | 0.34 | 1.7 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9.5 | 52.2 | 1.47 | 7.35 | |
| Salmonella ser. Senftenberg 775W | Liquid food product | Liquid egg whites | Heat | 9.5 | 55 | 0.36 | 1.8 | |
| Salmonella ser. Senftenberg 775W | Semi‐liquid food product | Chocolate | Heat | 70 | 440 | 2200 | ||
| Salmonella ser. Senftenberg 775W | Semi‐liquid food product | Chocolate | Heat | 71 | 276 | 1380 | ||
| Salmonella ser. Senftenberg 775W | Semi‐liquid food product | Chocolate | Heat | 80 | 116 | 580 | ||
| Salmonella ser. Senftenberg 775W | Semi‐liquid food product | Chocolate | Heat | 90 | 36 | 180 |
Table 4.
Identified intrinsic viral hazards in the ELS
| Material | Characteristics | Family | Viral hazard | Species reported to be affected | Reference from the viral hazard identification |
|---|---|---|---|---|---|
| Hides and skins | Enveloped DNA | Poxviridae | Orthopoxvirus | Cattle | Damaso et al. (2000) |
| Enveloped DNA | Poxviridae | Camelpox virus | Camelids | Otterbein et al. (1996), Balamurugan et al. (2013) | |
| Enveloped DNA | Poxviridae | Cutaneous avian poxvirus | Poultry | Ferreira et al. (2018) | |
| Enveloped DNA | Alloherpesviridae | Koi herpesvirus | Fish | Pokorova et al. (2005) | |
| Enveloped DNA | Poxviridae | Lumpy skin disease virus | Cattle, water buffalo | Biosecurity New Zealand (2007), Davies (1991), Carn (1993), Tuppurainen and Oura (2012), Tuppurainen et al. (2017), Abutarbush et al. (2016), Gelaye and Lamien (2019) | |
| Enveloped DNA | Herpesviridae | Marek's disease virus | Chicken | Jarosinski et al. (2007) | |
| Enveloped DNA | Poxviridae | Myxoma virus | Rabbit | Meredith (2013) | |
| Enveloped DNA | Poxviridae | Orf virus (Parapoxvirus) | Sheep, goats | Huerter et al. (1991), Haig and Mercer (1998), Haig and McInnes (2002), Lateef et al. (2010), Nandi et al. (2011), Fleming et al. (2015) | |
| Enveloped DNA | Poxviridae | Parapoxvirus | Deer | Ueda et al. (2007) | |
| Enveloped DNA | Poxviridae | Sheep poxvirus and Goat poxvirus | Sheep, goats | Biosecurity New Zealand (2007), Carn (1993), Babiuk et al. (2008), Tuppurainen et al. (2017) | |
| Enveloped RNA | Flaviviridae | Bovine viral diarrhoea virus (type 2)* | Cattle, sheep, pigs | Biosecurity New Zealand (2007), Grooms and Keilen (2002), Brodersen (2004), Babiuk et al. (2008) | |
| Enveloped RNA | Flaviviridae | Classical swine fever virus* | Pigs | Kaden et al. (2007) | |
| Enveloped RNA | Amnoonviridae | Tilapia lake virus | Fish | Behera et al. (2018) | |
| Non‐enveloped DNA | Papillomaviridae | Bovine papillomavirus | Equines, cattle | Borzacchiello et al. (2008), Taylor and Haldorson (2013), Trewby et al. (2014), Bocaneti et al. (2016) | |
| Non‐enveloped DNA | Papillomaviridae | Cottontail rabbit papillomavirus | Rabbit | Han et al. (1998) | |
| Non‐enveloped RNA | Reoviridae | Bluetongue virus | Sheep, cattle | MacLachlan et al. (2009) | |
| Non‐enveloped RNA | Picornaviridae | Foot and mouth disease virus* | Cloven‐hoofed animals | Biosecurity New Zealand (2007), Nfon et al. (2008) | |
| Non‐enveloped RNA | Picornaviridae | Swine vesicular disease virus | Pigs | Biosecurity New Zealand (2007) | |
| Wool and hair | Enveloped DNA | Poxviridae | Sheep and Goat poxviruses | Sheep, goats, Cattle | Zhou et al. (2012), Gale et al. (2016) |
| Enveloped RNA | Flaviviridae | Bovine viral diarrhoea* virus | Cattle | Singh et al. (2011), Callan et al. (2002) | |
| Non‐enveloped RNA | Picornaviridae | Foot and mouth disease* virus | Sheep | McColl et al. (1995) | |
| Non‐enveloped DNA | Parvoviridae | Ungulate tetraparvovirus | Mule deer | Li et al. (2016) | |
| Enveloped DNA | Poxviridae | Orf virus (Parapoxvirus) | Sheep | Fleming et al. (2017) | |
| Enveloped RNA | Flaviviridae | Border disease virus (BDV) | Sheep | Kalaiyarasu et al. (2019) | |
| Feathers and down | Non‐enveloped DNA | Anelloviridae | Chicken anaemia virus | Poultry | Todd (2000), Davidson and Skoda (2005), Hernandez‐Divers et al. (2006) |
| Non‐enveloped DNA | Circoviridae | Duck circovirus | Duck | Liu et al. (2020) | |
| Enveloped RNA | Orthomyxoviridae | Avian influenza virus* | Poultry | Kaleta and Hönicke (2004), Hafez (2005), Dudley (2008), Beato et al. (2009), Yamamoto et al. (2010, 2017), Huchzermeyer (1997) | |
| Enveloped RNA | Paramyxoviridae | Newcastle disease virus* | Poultry | Hafez (2005), Huchzermeyer (1997), Hernandez‐Divers et al. (2006) | |
| Enveloped DNA | Herpesviridae | Marek′s disease virus | Poultry | Couteaudier and Denesvre (2014), Couteaudier et al. (2016), Zhang et al. (2015), Davidson et al. (2005); Denesvre (2013) | |
| Enveloped DNA | Poxviridae | Fowlpox virus | Poultry | Davidson and Skoda (2005) | |
| Enveloped RNA | Retroviridae | Reticuloendotheliosis virus | Poultry | Davidson and Skoda (2005) | |
| Enveloped RNA | Retroviridae | Avian leucosis virus | Poultry | Davidson and Skoda (2005) | |
| Enveloped RNA | Flaviviridae | West Nile virus | Avian carcasses | Nemeth et al. (2009) | |
| Pig bristles | None reported | ||||
| Horns, horn products, hooves and hoof products | Non‐enveloped RNA | Picornaviridae | Senecavirus A | Pigs, cattle | Niedbalski and Fitzner (2019) |
viral hazards for which thermal inactivation kinetics or other relevant information was already available (REFRESH study: Hayrapetyan et al., 2019).
2.3. Thermal and chemical inactivation (AQ2, AQ4, AQ5)
An extensive literature search was conducted to extract data from the scientific literature on thermal and chemical inactivation of E. faecalis and S. Senftenberg in the form of time/temperature/pH combinations in defined matrices (even if different from those in the mandate). Data on chemical inactivation of S. Senftenberg were extracted from this extensive literature review, whereas data on thermal inactivation of this bacteria were extracted from the review of studies on the thermal resistance of salmonellae executed by Doyle and Mazzotta (2000).
An extensive literature search was conducted to extract data from the scientific literature on thermal and chemical inactivation of the selected viruses in the form of time/temperature/pH combinations in defined matrices (even if different from the ones in the mandate). Out of the virus identified as hazards in the extensive literature search (ELS) described in Section 2.2 to address AQ3, only non‐enveloped viruses that may be intrinsically present in the materials were selected as the most resistant to thermal and other treatments for each group.
An ELS was conducted to extract data from the scientific literature on chemical inactivation of Ascaris spp. in the form of time/temperature/pH combinations in defined matrices (even if different from those in the mandate).
The description of these three ELS is detailed below.
The criterion for selection of references was experimental studies in which resistance parameters (D‐ and/or Z‐values) or the levels of reduction or inactivation of the selected bacteria, viruses or parasites (preferably measured in log10) had been measured after thermal and/or chemical treatment in matrices preferably similar to the ones included in the mandate. The database used was Scopus, Elsevier (English language, worldwide and not restricted in years).
The search strings were designed by combining the generic names of biological hazards (selected bacteria, viruses or parasites) AND (inactivation method) AND inactivation. The criteria for inclusion were if in the title, abstract or keywords, the specific name of the hazard (indicator microorganism or biological hazard) and inactivation/resistance keywords were mentioned. Details of the search strings can be found in Table A.1 of Annex A.
Table C.1.
Summary of the rationale for the consensus of each combination, as recorded by the rapporteur
| Process | Summary of rationale as recorded by the rapporteur. |
|---|---|
| 1.1 Ash derived from incineration 850°C, > 2 s | Although the temperature of the process (850°C) is extremely high and the three indicators would be reduced to the desired level, there is some uncertainty left considering that the duration of 2 seconds may not be sufficient, the starting concentration is unknown and there may be some protecting material. |
| 1.2 Ash derived from incineration1,100°C, > 0.2 s | The temperature of the process (1,100°C) is higher than in 1.2 but with shorter time (0.2 s) and it is believed that the three indicators would be reduced to the desired level. Still, the starting concentration is unknown and there may be some protecting material leaving some uncertainty. |
| 2.1 Glycerine derived from the production of biodiesel and renewable fuels Category 2 materials 133°C, 20 min, 3 bar (Method 1) + pH < 1/72°C/> 2 h (esterification) + pH ˜ 14/35°C to 50°C/> 15 min (transesterification) | The thermal treatment (133°C, 20 min, 3 bar) alone would give significant reductions of the three indicators as inactivation of non‐spore forming bacteria at temperatures > 100°C should be achieved in a few seconds. There is a sequence of this thermal with chemical treatments (esterification and transesterification) that is expected to give further reductions. There is some uncertainty left as the material is pure fat in which it is more difficult to reduce the three indicators. |
| 2.2 Glycerine derived from the production of biodiesel and renewable fuels Category 3 materials 80°C, 120 min (Method 5 (a)) + pH ˜ 14/35°C to 50°C/> 15 min (transesterification) | As in 2.1, it needs to be considered that the material is pure fat. The probability ranges of E. faecalis and S. Senftenberg are considered the same. The range is quite broad reflecting the uncertainty on whether there is a difference or not; In case there was a difference, there would be a bit lower confidence for S. Senftenberg than for E. faecalis. The probability for the Parvovirus is lower compared to the bacterial indicators as the thermal treatment may be effective but there is no clear indication on the effect of the pH on the virus inactivation during the transesterification step. |
| 2.3 Glycerine derived from the production of biodiesel and renewable fuels Category 3 materials 100°C 60 min (Method 5(b)) + pH ˜ 14/35°C to 50°C/> 15 min (transesterification) | The rationale is the same as in 2.2. The probability range has been increased compared to 2.2 for the bacterial indicators and the Parvovirus because of the higher temperature used in this process for which there is more evidence of inactivation. |
| 3.1 Other products of materials derived from the production of biodiesel and renewable fuels Category 2 materials Method 1: 133°C, 20 min, 3 bar | This is the same process as in 2.1 but without transesterification and with a different material (but with high fat content). There is some evidence that the treatment would be less effective on S. Senftenberg compared to E. faecalis. However, this difference is not big enough to have a different probability range (i.e. E. faecalis a bit higher and S. Senftenberg a bit lower). For the Parvovirus, the range was considered the same as for the bacteria considering the evidence on thermal inactivation. |
| 3.2 Other products of materials derived from the production of biodiesel and renewable fuels Category 3 materials Method 5(a): 80°C, 120 min | The process is the same as in 3.1, but with lower temperature and higher time. It is also the same process as in 2.2 but without transesterification and with a different material (but with high fat content). The evidence on thermal inactivation indicates that the thermal treatment is not sufficient to inactivate S. Senftenberg. Compared to 3.1, the difference between S. Senftenberg and E. faecalis is more obvious as there is a larger range here. For the Parvovirus there is more uncertainty here based on the available evidence on thermal inactivation. |
| 3.3 Other products of materials derived from the production of biodiesel and renewable fuels Category 3 materials Method 5(b): 100°C, 60 min | The rationale is the same as in 3.2. The lower bound of the probability range has been increased compared to 3.2 for the bacteria and the Parvovirus because of the higher temperature used in this process in which there is more evidence for inactivation. The difference between the bacterial indicators is again more obvious as the range is larger. |
| 4.1 Hides and skins Limed hides pH 12–13, 8 h | There is less evidence about the inactivation for this process and there is uncertainty about the level of heat released through the liming process which is dependent on the lime concentration used (concentration or temperature are not mentioned in legislation). The desired reduction may not be achieved using the pH alone also considering that the matrix has a very low water content. For Ascaris the time needed for inactivation (as in studies) is much longer. For the viruses (Papillomavirus, Picornavirus, Reovirus), the reduction is more difficult to achieve compared to bacterial indicators, but easier compared to Ascaris. |
| 4.2 Hides and skins pH ˜ 12, > 8 h + pH < 3, 16 h | Compared to 4.1, the matrix is the same, but a treatment has been added (pH < 3 16 h) and the alkaline treatment lasts longer. The upper bound has been increased for the bacteria considering the acid treatment. There is little information on the inactivation of Ascaris eggs at acidic pH which may add an additional reduction. For the viruses, there is less information available than for bacterial indicators but there is some evidence for reductions at acidic pH values. |
| 5.1 Wool and hair pH > 12–13, 5 min | There are no data available for such short alkaline processes which may be too short to achieve the desired level of reduction. The temperature will not rise because the processing time is short. Ascaris eggs are likely more difficult to reduce by alkaline processes than the bacterial indicators and viruses. |
| 5.2 Wool and hair pH > 12–13, 60 min | As the treatment time is longer compared to 5.1, both the lower and upper ranges have been increased. |
| 6. Feather and down 100°C for at least 30 min | The treatment is made with steam and after washing, which would increase the water content of the matrix. The temperature/time combination would be sufficient to inactivate the bacterial indicators. For the viruses (Anellovirus, Circovirus), there is higher uncertainty than for bacteria. |
| 7.1 Pig bristles 100°C in water, 5 min | The treatment is made with boiling water, which would increase the water content of the matrix. The time is shorter compared to 6, and both the lower and upper ranges have been decreased. For parvoviruses, contradictory data are available around 100°C. |
| 7.2 Pig bristles 100°C in water, 60 min | The treatment is at the same temperature as 7.1 but for longer time and therefore the lower and upper ranges have been increased. |
| 8. Horns, horn products, hooves and hoof products 80°C 1 h | The material is rich in protein and with a very low‐fat content and lower water activity than other materials, which led to an increase of the uncertainty. Picornaviridae are less resistant than Parvoviridae and the evidence mainly supports that the desired reduction would be achieved. |
The selection of studies was made by screening the title and abstract of the references extracted following the application of the search strings. The list of references was presented in tabular format in a template containing the following fields: Authors, Title, Year, DOI, Link and Abstract. The list of references for each indicator microorganism or biological hazard was screened by WG members and EFSA staff. For each reference, one of the following three options was assigned: ‘Yes’, when it was possible to extract inactivation data by looking at the paper in full; ‘Doubtful’, when it may be useful in a second round to look more in depth; ‘No’ when the reference was to be discarded. References in category Yes were retrieved and full papers were reviewed, splitting them into two groups: those with/without relevant data to be extracted. Data from papers with relevant data were transferred to a tabular template including the preselected set of fields. Review of doubtful references was left at the discretion of the reviewer.
Data extracted on thermal inactivation were presented in tabular format using a template with the following fields: hazard (virus, etc.), matrix/substrate, initial load, treatment, temperature (°C), time (min), level of inactivation, D‐value (min) and reference. Data extracted on chemical inactivation were presented in tabular format using a template with the following fields: matrix/substrate, indicator microorganism/biological hazard, initial load, treatment, level of inactivation and reference.
The data obtained from the literature were considered by the WG to evaluate, using EKE, the certainty on whether the required level of reduction is achieved for the indicator microorganisms and/or biological hazards by the standard processing parameters identified in AQ1 and for each of the materials.
2.4. Uncertainty analysis and expert knowledge elicitation
Based on the EFSA guidance on Uncertainty Analysis in Scientific Assessments (EFSA Scientific Committee, 2018a) and the scientific opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018b), the sources of uncertainty associated with the available data were summarised in tabular format (Table 12 in Section 4), describing also the cause of the uncertainty. The impact of the uncertainty on the level of inactivation of the selected indicator microorganisms and biological hazards was described, without specifying in which of the combinations of material, process and hazard, the over‐/underestimation would occur.
An EKE was performed to answer AQ2, AQ4 and AQ5, based on the collected evidence and indicated uncertainties. The EKE questions were specified as follows:
What is the probability that a 5 log10 reduction of E. faecalis is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process(es) is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 5 log10 reduction of S. Senftenberg (775W, H2S negative) is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 3 log10 reduction of parvovirus or the identified most resistant viruses is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 3 log10 reduction of eggs of Ascaris sp. is achieved, in more than 99% of cases, by application of the relevant chemical process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
In these questions, the phrase ‘in more than 99% of cases’ refers to the potential variation in the performance of the relevant process/es. As the process/es is/are well defined, this variation is considered to be small. The ‘probability’ refers to the certainty that the log10 reduction is achieved if this well‐defined process is performed.
The EKE consisted of two steps: individual judgements and consensus judgements. In Step 1, the experts provided individual judgements for each of the 52 combinations of material/process/hazard by considering them separately, taking into account the version of the draft opinion at the beginning of the process with the raw data on thermal and chemical inactivation of the indicators, the description of the processes, the integration of the evidence and the uncertainty table, as well as the personal expertise and assessment of the uncertainties involved. In Step 2, during an open session, the experts were asked to consider what a rational impartial observer (RIO) would judge, having considered the evidence, uncertainties, the individual judgements and having heard the discussion maintained by the experts. As the starting point for the discussions, a potential consensus probability range was proposed by the facilitator, based on the mean of the median estimates of all the individual ranges. The objective of Step 2 was to reach consensus on the probability ranges that were considered to best represent the uncertainty on whether the indicated log10 reductions are achieved with the standard processes for the different materials. Detailed information on the EKE can be found in Appendix C and in Table A.1 of Annex A.
3. Assessment
3.1. Introduction
3.1.2 Category 2 and 3 animal by‐products and derived products
Regulation (EC) No 1069/2009 defines ‘animal by‐products’ as ‘entire bodies or parts of animals, products of animal origin or other products obtained from animals, which are not intended for human consumption, including oocytes, embryos and semen; and ‘derived products’ as products obtained from one or more treatments, transformations or steps of processing of animal by‐products.’
The use or fate of ABP depends on their risk classification in three different categories: (i) Category 1 material consists mainly of material that is considered at transmissible spongiform encephalopathy (TSE) risk, and as such represents the highest risk material; (ii) category 2 material includes fallen stock, manure and gastrointestinal contents; (iii) Category 3 materials are considered of a lower risk level and include parts of animals that have been considered fit for human consumption at the slaughterhouse, but that are not intended for human consumption for production or commercial reasons (e.g. trimmings of carcasses, consumer rejection to certain organs, etc.).
More specifically, and within the framework of this assessment, Article 9 of Regulation (EC) No 1069/2009 lists as Category 2 materials the following ABP:
manure, non‐mineralised guano and digestive tract content;
-
animal by‐products collected during the treatment of waste water required by implementing rules adopted under point (c) of the first paragraph of Article 27:
from establishments or plants processing Category 2 material;
or from slaughterhouses other than those covered by Article 8(e);
animal by‐products containing residues of authorised substances or contaminants exceeding the permitted levels as referred to in Article 15(3) of Directive 96/23/EC;
products of animal origin which have been declared unfit for human consumption due to the presence of foreign bodies in those products;
-
products of animal origin, other than Category 1 material, that are:
imported or introduced from a third country and fail to comply with Community veterinary legislation for their import or introduction into the Community except where Community legislation allows their import or introduction subject to specific restrictions or their return to the third country; or
dispatched to another Member State and fail to comply with requirements laid down or authorised by Community legislation except where they are returned with the authorisation of the competent authority of the Member State of origin;
-
animals and parts of animals, other than those referred to in Article 8 or Article 10,
that died other than by being slaughtered or killed for human consumption, including animals killed for disease control purposes;
foetuses;
oocytes, embryos and semen which are not destined for breeding purposes; and
dead‐in-shell poultry;
mixtures of Category 2 material with Category 3 material;
animal by‐products other than Category 1 material or Category 3 material (Article 9, Regulation (EC) No 1069/2009).
In relation to Category 3 materials, Article 10 lists among them the following ABP relevant to this mandate:
carcases and parts of animals slaughtered or, in the case of game, bodies or parts of animals killed, and which are fit for human consumption in accordance with Community legislation, but are not intended for human consumption for commercial reasons;
-
carcasses and the following parts originating either from animals that have been slaughtered in a slaughterhouse and were considered fit for slaughter for human consumption following an ante‐mortem inspection or bodies and the following parts of animals from game killed for human consumption in accordance with Community legislation:
carcasses or bodies and parts of animals which are rejected as unfit for human consumption in accordance with Community legislation, but which did not show any signs of disease communicable to humans or animals
heads of poultry;
-
hides and skins, including trimmings and splitting thereof, horns and feet, including the phalanges and the carpus and metacarpus bones, tarsus and metatarsus bones, of:
-
–
animals, other than ruminants requiring TSE testing, and
-
–
ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No 999/2001;
-
–
pig bristles
feathers
-
c
animal by‐products from poultry and lagomorphs slaughtered on the farm as referred to in Article 1(3)(d) of Regulation (EC) No 853/2004, which did not show any signs of disease communicable to humans or animals;
-
d
blood of animals which did not show any signs of disease communicable through blood to humans or animals obtained from the following animals that have been slaughtered in a slaugh−terhouse after having been considered fit for slaughter for human consumption following an ante‐mortem inspection in accordance with Community legislation:
animals other than ruminants requiring TSE testing; and
ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No 999/2001;
-
e
animal by‐products arising from the production of products intended for human consumption, including degreased bones, greaves and centrifuge or separator sludge from milk processing;
-
f
products of animal origin, or foodstuffs containing products of animal origin, which are no longer intended for human consumption for commercial reasons or due to problems of manu−facturing or packaging defects or other defects from which no risk to public or animal health arise;
-
g
petfood and feeding stuffs of animal origin, or feeding stuffs containing animal by‐products or derived products, which are no longer intended for feeding for commercial reasons or due to problems of manufacturing or packaging defects or other defects from which no risk to public or animal health arises;
-
h
blood, placenta, wool, feathers, hair, horns, hoof cuts and raw milk originating from live animals that did not show any signs of disease communicable through that product to humans or animals;
-
i
aquatic animals, and parts of such animals, except sea mammals, which did not show any signs of disease communicable to humans or animals;
-
j
animal by‐products from aquatic animals originating from establishments or plants manufacturing products for human consumption;
-
k
the following material originating from animals which did not show any signs of disease communicable through that material to humans or animals:
shells from shellfish with soft tissue or flesh;
the following originating from terrestrial animals: — hatchery by‐products, — eggs, — egg by‐products, including egg shells,
day‐old chicks killed for commercial reasons;
-
l
aquatic and terrestrial invertebrates other than species pathogenic to humans or animals;
-
m
animals and parts thereof of the zoological orders of Rodentia and Lagomorpha, except Category 1 material as referred to in Article 8(a)(iii), (iv) and (v) and Category 2 material as referred to in Article 9(a) to (g);
-
n
hides and skins, hooves, feathers, wool, horns, hair and fur originating from dead animals that did not show any signs of disease communicable through that product to humans or animals, other than those referred to in point (b) of this Article;
-
o
adipose tissue from animals which did not show any signs of disease communicable through that material to humans or animals, which were slaughtered in a slaughterhouse and which were considered fit for slaughter for human consumption following an ante‐mortem inspection in accordance with Community legislation;
-
p
catering waste other than as referred to in Article 8(f).
3.1.2 End points in the manufacturing chain
Article 5(2) of Regulation (EC) 1069/2009 states that: ‘for derived products referred to in articles 32 (organic fertilisers and soil improvers), 35 (pet food) and 36 (other derived products), which no longer pose any significant risk to public or animal health, an end point in the manufacturing chain may be determined, beyond which they are no longer subject to the requirements of this Regulation’.
Article 32 refers to organic fertilisers and soil improvers which may be placed on the market and used under certain conditions. In addition, digestate, the by‐product from the transformation of food waste, sludge, animal slurry, grease‐trap waste, etc. into biogas or compost may be placed on the market and used as organic fertiliser or soil improver.
Article 35 refers to pet food under certain conditions.
Article 36 refers to other derived products than the products referred to in Articles 31, 32, 33 and 35. Those derived products may subsequently be placed on the market without restrictions under Regulation (EC) 1069/2009 and shall no longer be subject to official controls in accordance with Regulation (EC) 1069/2009.
The legal context of this mandate is specified in point 4 of Article 5, as amended by Regulation (EU) 2019/1009, which states that: ‘within six months after 15 July 2019, the Commission shall initiate a first assessment of derived products referred to in Article 32 that are already widely used in the Union as organic fertilisers and soil improvers. This assessment shall cover at least the following products: meat meal, bone meal, meat‐and-bone meal, hydrolysed proteins of Category 3 materials, processed manure, compost, biogas digestion residues, feather meal, glycerine and other products of Category 2 or 3 materials derived from the production of biodiesel and renewable fuels, as well as petfood, feed and dog chews that have been refused for commercial reasons or technical failures, and derived products from blood of animals, hides and skins, hoofs and horns, guano of bats and birds, wool and hair, feathers and downs, and pig bristles. Where the assessment concludes that those derived products no longer pose any significant risk to public or animal health, the Commission shall determine an end point in the manufacturing chain pursuant to paragraph 2 of this Article without undue delay and in any case no later than six months after the assessment is finalised.’
Commission Regulation (EU) No 142/201121 lays down implementing measures: (a) for the public and animal health rules for animal by‐products and derived products laid down in Regulation (EC) No 1069/2009; (b) concerning certain samples and items exempt from veterinary checks at border inspection posts as provided for in Article 16(1)(e) and (f) of Directive 97/78/EC. This EU regulation provides requirements regarding the processing and transformation of ABP into different derived materials. In relation to the declaration of end points, there are a number of derived products that have been declared end points in the manufacturing chain according to this regulation. Thus, article 3 of Commission Regulation (EU) 142/2011 lists the derived products that may be placed on the market, other than imported, without restrictions, as provided in Article 5(2) of Regulation (EC) No 1069/2009:
biodiesel which fulfils the requirements for the disposal and use of derived products set out in point 2(b) of Section 3 of Chapter IV of Annex IV;
processed petfood which fulfils the specific requirements for processed petfood set out in point 7(a) of Chapter II of Annex XIII;
dog chews which fulfil the specific requirements for dog chews set out in point 7(b) of Chapter II of Annex XIII;
hides and skins of ungulates which fulfil the specific requirements for the end point for those products set out in point C of Chapter V of Annex XIII;
wool and hair, which fulfil the specific requirements for the end point for those products set out in point B of Chapter VII of Annex XIII;
feathers and down, which fulfil the specific requirements for the end point for those products set out in point C of Chapter VII of Annex XIII;
fur which fulfils the special requirements for the end point for that product set out in Chapter VIII of Annex XIII;
fish oil for the production of medicinal products which fulfils the special requirements for the end point for that product set out in Chapter XIII of Annex XIII;
gasoline and fuels which fulfil the specific requirements for products from the multi‐step catalytic process for the production of renewable fuels set out in point 2(c) of Section 3 of Chapter IV of Annex IV;
oleochemical products derived from rendered fats and which fulfil the requirements set out in Chapter XI of Annex XIII;
renewable diesel, renewable jet fuel, renewable propane and renewable gasoline which fulfil the specific requirements for products from the multi‐step catalytic hydro‐treatment for the production of renewable fuels set out in point 2(f) of Section 3 of Chapter IV of Annex IV.
3.2. Description of the materials included in the mandate
3.2.1. Ash derived from incineration, co‐incineration and combustion
According to the ABP EU Regulations, incineration can be defined as the disposal of ABP or derived products as waste, in an incineration plant, as defined in point 4 of Article 3 of Directive 2000/76/EC22. Co‐incineration means the recovery or disposal of ABP or derived products, if they are waste, in a co‐incineration plant. Finally, combustion means a process involving the oxidation of fuel in order to use the energy value of the ABP or derived products, if they are not waste.
According to Regulation (EC) 1069/2009, ABP, either categorised as Cat. 1, Cat. 2 or Cat. 3 material, can be disposed of as a waste by incineration or co‐incineration directly without prior processing or following processing, by pressure sterilisation, if the competent authority requires so, and permanent marking of the resulting material, or used as a fuel for combustion with or without prior processing.
The process can take place in open‐air, fixed‐facility or air‐curtain systems. Open‐air systems include the burning of carcasses or other ABP on combustible heaps known as pyres. Material requirements for open‐air burning include straw or hay, untreated timbers, kindling wood, coal and diesel fuel. Fixed‐facility systems include (a) small on‐farm incinerators, (b) small and large incineration facilities, (c) crematoria and (d) power plant incinerators. Fixed‐facility systems are wholly contained and, usually, highly controlled. They are typically fuelled by diesel, natural gas or propane. Newer designs of fixed‐facility systems are fitted with afterburner chambers designed to completely burn hydrocarbon gases and particulate matter exiting from the main combustion chamber. In air‐curtain systems, large‐capacity fans, driven by diesel engines, deliver high‐velocity air down into either a metal refractory box or burn pit (trench). Air‐curtain systems vary in size according to the amount of material to be incinerated. Materials needed for air‐curtain systems include wood (preferably pallets) and fuel (e.g., diesel fuel) for both the fire and the air‐curtain fan. Unlike fixed‐facility systems, air‐curtain systems are not wholly contained and are at the mercy of many variable factors (e.g. human operation, the weather, local community preferences, etc.) (National Agricultural Biosecurity Center, 2004).
According to Annex III, Chapter 1, Section 2 of Commission Regulation (EU) No 142/2011, incineration or co‐incineration plants shall be designed, equipped, built and operated in such a way that the gas resulting from the process is raised in a controlled and homogeneous fashion, even under the most unfavourable conditions, to a temperature of 850°C for at least 2 s or to a temperature of 1,100°C for 0.2 s, as measured near the inner wall or at another representative point of the chamber where the incineration or the co‐incineration is carried out, as authorised by the competent authority.
3.2.2. Glycerine derived from the production of biodiesel and renewable fuels
Biodiesel consists of mono‐alkyl esters of long‐chain fatty acids mostly produced from vegetable oils and animal fats. The total global production of biodiesel was approximately 35–45 million tonnes in 2019 (Flach et al., online). The European Union is the world's largest biodiesel producer and, in 2020, the European biodiesel industry produced in 2020 more than 15 million tonnes of biodiesel (75% of the total transport biofuels market on an energy basis).23
For biodiesel production, a fat fraction derived from ABP of all categories may be used. Such fats include extracted beef tallow, mutton tallow, pork lard and chicken fat (Sai Akhil and Alagumalai, 2019). Other fats used are those resulting from meat and the meat processing industry and those from recycling practices within the industrial cooking business. In 2019, 800,000 thousand tonnes (˜ 6% of total feedstock) corresponded to animal fats, and such amount has remained fairly constant since 2014 (Ramos et al., 2019).
The major steps in the production of biodiesel from animal fat waste are shown in Figure 1. A pretreatment (e.g. heat drying, silica gel, treatment with calcium chloride or anhydrous sodium sulfate, neutralisation or separation) is needed because feedstocks usually contain a high proportion of free fatty acids (FFA) and water which reduce the yield of biodiesel and have to be removed (Gebremariam and Marchetti, 2018; Felizardo et al., 2006; Lee et al., 2002). Biodiesel is then produced through a transesterification reaction of a fat with a short‐chain alcohol (usually methanol) in the presence of a catalyst (such as an alkali, acid or enzyme) (Ma and Hanna, 1999; Ramadhas et al., 2005). Transesterification consists of the conversion of triacylglycerols to diacylglycerols, releasing one fatty acid. Diacylglycerols are then converted to monoacylglycerols, releasing a second fatty acid and, finally, monoacylglycerols are converted to glycerol, releasing a third fatty acid (Toldrá‐Reig et al., 2020). In industrial processing plants, ˜ 100 kg of fat react with 10 kg of methanol in the presence of an alkaline catalyst (i.e. sodium hydroxide or potassium hydroxide), to produce 100 kg of biodiesel and 10 kg of glycerine (US Department of Energy, 2020; Toldrá‐Reig et al., 2020).
Figure 1.
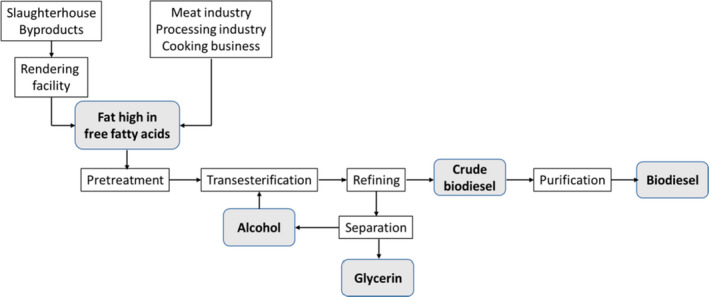
Major steps in the production of biodiesel from animal fat waste (Toldrá‐Reig et al., 2020)
An alternative process would be a two‐step transesterification, with the first step being an acid‐catalysed pretreatment to esterify the FFA and thus reduce their content, and the second step being the transesterification (Ramadhas et al., 2005).
Large amounts of glycerine (containing at least 95% glycerol) are generated during transesterification. Glycerine is purified, along with the removal of other impurities such as residual catalyst, unconverted fats and soap, through wet washing, based on water, dry washing, based on adsorption and ion exchange, or novel methods based on liquid–liquid extraction, deep eutectic solvents or membranes (Sander et al., 2018). Glycerine is a versatile and valuable chemical substance with many applications. In the conventional glycerine refining processes, the crude glycerine solution is initially treated with additional chemicals to remove any dissolved fatty acids or soaps, followed by processing in a higher temperature, high vacuum distillation unit. The condensed glycerine solution is further treated to remove traces of residual fatty acids, esters or other organic compounds (FAO, 2012).
According to Annex IV, Chapter IV, Section 2 of Commission Regulation (EU) No 42/2011, biodiesel production shall be carried out according to the following processing standards:
-
Unless fish oil or rendered fat are used which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No 853/2004, respectively, the fat fraction derived from animal by‐products must be first processed using:
in the case of Category 1 or 2 materials, processing method 1 (pressure sterilisation) as set out in Chapter III; and
in the case of Category 3 materials, any of the processing methods 1–5 or processing method 7 or, in the case of material derived from fish, processing methods 1–7 as set out in Chapter III;
-
The processed fat must then be processed further using one of the following methods:
a process whereby the processed fat must be separated from the protein and in the case of fat from ruminant origin, insoluble impurities in excess of 0.15% by weight must be removed, and the processed fat must be subsequently submitted to esterification and transesterification. However, esterfication is not required for processed fat derived from Category 3 material. For esterfication the pH must be reduced to less than 1 by adding sulphuric acid (H 2 SO 4 ) or an equivalent acid and the mixture must be heated to 72°C for at least two hours during which it must be intensely mixed. Transesterfication must be carried out by increasing the pH to about 14 with potassium hydroxide or with an equivalent base at 35°C to 50°C for at least 15 min. Transesterfication shall be carried out twice under the conditions described in this point using a new base solution. This process must be followed by refinement of the products including vacuum distillation at 150°C, leading to biodiesel;
a process using equivalent process parameters authorised by the competent authority.
3.2.3. Other materials derived from the production of biodiesel and renewable fuels
Other renewable fuels (jet fuel, propane, gasoline) can also be obtained from the same sort of feedstocks (i.e. rendered fats) used to produce biodiesel through a multistep process involving a pretreatment which consists of bleaching and removal of remaining insoluble impurities by filtration, followed by a catalytic conversion step at high temperatures (250–265°C) and high pressures (20–30 bar). The main by‐products generated in the pretreatment process are clay from bleaching and sludge from filtration.
Annex IV, Chapter IV, Section 2 of Commission Regulation (EU) No 142/2011 highlights that some renewable fuels can be produced through alternative methods:
Point 2.J: multistep catalytic process
-
The starting materials for this process can be:
rendered fats derived from Category 2 material, which have been processed using processing method 1 (pressure sterilisation);
Fish oil or rendered fats derived from Category 3 material, which have been processed using: any of the processing methods 1–5 or processing method 7; or in the case of material derived from fish oil, any of the processing methods 1–7;
Fish oil or rendered fat which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No 853/2004, respectively.
The use of rendered fats derived from Category 1 material for this process shall be prohibited.
According to point 2.J.2, the process consists of:
-
A pre‐treatment which consists of:
the bleaching of the centrifuged materials by passing them through a clay filter;
the removal of remaining insoluble impurities by filtration.
The pre‐treated materials must be then submitted to a multi‐step catalytic process which consists of a hydro‐deoxygenisation step, followed by an isomerisation step. The materials must be submitted to a pressure of at least 20 bars at a temperature of at least 250°C for at least 20 min.
Point 2.L: multistep catalytic hydro‐treatment process:
Point 2.L.1 describes the pretreatment. For this process, the following materials may be used:
rendered fats derived from Category 1 material, which have been processed using processing method 1 (pressure sterilisation);
rendered fats and fish oil complying with point J(1)(a) of this Section.
According to point 2.J.2:
The rendered fat must be submitted to a pre‐treatment which consists at least of bleaching of the starting material, including rendered fats, with acid in the presence of bleaching clay and subsequent removal of the used bleaching clay and insoluble impurities by filtration.Prior to this treatment rendered fat may be degummed with acid and/or caustic solution in order to remove impurities from the rendered fat by forming gums and subsequently separating those gums by centrifugation.
The pre‐treated materials must be then submitted to a hydro‐treatment process which consists of a catalytic hydro‐treatment step, a stripping step followed by an isomerisation step. The materials must be submitted to a pressure of at least 30 bars at a temperature of at least 265°C for at least 20 min.
The raw materials for both methods are rendered fats processed by the different methods of ABP processing according to Commission Regulation (EU) 142/2011 (Appendix B) before they are subject to the pretreatments, resulting in the production of derived products. Thus, the subsequent hydrolytic processes to produce renewable fuels do not affect the derived products like bleaching clay.
3.2.4. Hides and skins
As already mentioned in Section 3.1, point 3 Article 10 of Regulation (EC) 1069/2009 defines as Category 3 ABP: ‘hides and skins, including trimmings and splitting thereof, […] of: animals, other than ruminants requiring TSE testing, and ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No 999/2001, as well as hides and skins, […] originating from dead animals that did not show any signs of disease communicable through that product to humans or animals, other than those referred to in (b) of this Article.’
Hides and skins are one of the most valuable ABP of the meat industry. Most of the raw material is converted into leather and processed products such as collagen, gelatine, protein hydrolysates, pet chews or glue.
The skin is composed of three major layers: the surface pigmented epidermis including the top layer of the hide (grain), the underlying connective tissue between the grain and the corium and the deep subcutis (corium) (Figure 2). The main component (over 53%) of hides is protein (Bwirhonde et al., 2018), represented by multiple components such as albumin, elastin, collagen and keratin.
Figure 2.
Hides and/or skins can be obtained from all species of farmed animals including bovines, small ruminants, swine, equines, poultry, cervids and fish, for multiple purposes. They can either be obtained from slaughterhouses directly or their subproducts from tanneries or leather producing facilities. The skins of heads and legs are not removed from the bones and are usually disposed of and rendered together, not being harvested and used as stand‐alone by‐products.
The processing of hides and skins generates by‐products such as hide offcuts and shavings, or soft material from fleshing, which find applications in several industry sectors such as pet and animal food production, fine chemicals including photography and cosmetics, and fertilisers (European Commisssion, online; JRC, 2013). For use as organic fertilisers, different end products can be obtained, depending on the transformation process: enzymatic hydrolysis (liquid fertiliser) and thermal hydrolysis (solid fertiliser) (Ciavatta et al., 2012).
In the leather industry, approximately 70% of the weight of fresh raw materials is solid waste, most of it produced during the pre‐tanning or beam house process (80%). Solid waste generated by the leather industry during tannery and post‐tannery can be classified as follows (Ozgunay et al., 2007): (i) wastes from untanned hides/skins (trimmings, fleshing wastes); (ii) wastes from tanned leather (shaving wastes, buffing dust); and (iii) wastes from dyed and finished leather (trimmings from leather).
Fresh hides and skins need to be preserved for collection, transport and further processing. The main goal is to remove the moisture (either by air or with salt) to protect the hides and skins from putrefaction. This process is called curing and leads to a more stable material (FAO, 1996).
According to the definitions of Annex I of Commission Regulation (EU) No 142/2011: ‘treated hides and skins means derived products from untreated hides and skins, other than dog‐chews, that have been: (a) dried; (b) dry‐salted or wet‐salted for a period of at least 14 days prior to dispatch; (c) salted for a period of at least seven days in sea salt with the addition of 2% of sodium carbonate; (d) dried for a period of at least 42 days at a temperature of at least 20°C; or (e) subject to a preservation process other than tanning.’
As the hides and skins that may be placed on the market without restrictions refer to those for purposes other than human consumption and to those treated and destined for the leather industry, details of the processing of hides and skins for the production of leather are described below.
Manufacturing of leather is a multistep process (Figure 3), extensively described in technical and scientific literature. The main processing steps are beamhouse operations, tanning, post‐tanning and finishing. Solid waste is generated mostly pre‐tanning at the beamhouse.
Figure 3.
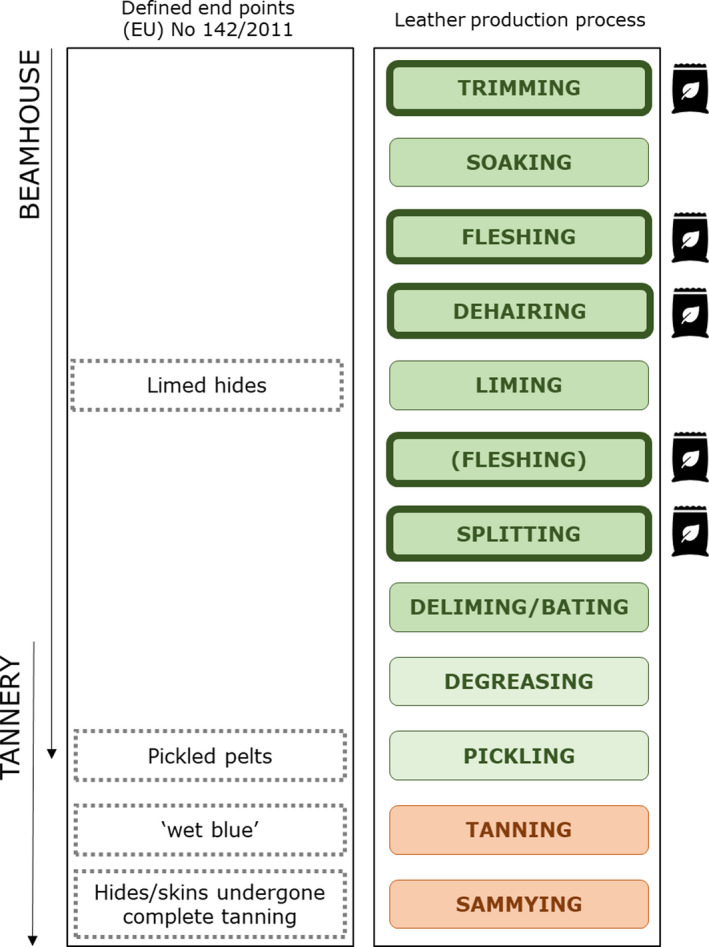
Steps of the processing of hides and skins for the leather production
Beamhouse
The beamhouse stage includes all the operations to prepare the raw material for tanning. As described by Ozgunay et al. (2007), Chattopadhyay et al. (2011), Valeika et al. (2012), FAO (1996), Biosecurity New Zealand (2007) and ENEA (2020), it commonly includes:
Trimming: mechanical removal of unwanted parts.
Soaking: re‐hydration and washing to remove substances like dirt, blood and salt (a bactericide can be added).
Fleshing: mechanical removal of excess flesh and fat (hypodermis) adhered to the hide. The material obtained by removing meat residues and connective subcutaneous tissues from the flesh side of the skin before further processing is considered one of the most important by‐products from the leather industry and accounts for about 10–15% of the animal skin (Corte et al., 2014) and up to 30% of the solid waste (Chattopadhyay et al., 2011). To ensure even thickness, excess material from the flesh side of the hide is removed (shaving).
Dehairing: immersion in an alkaline solution [lime (Ca(OH)2) in combination with sulfides such as sodium sulfide (Na2S) (pH > 12)] to remove the epidermis and the hair and wool and to loosen the fibrous structure of the hide. This process can be of different durations based on the combination of chemicals used in the process; from minutes to hours (Valeika et al., 2012). The hair can be used further for fertiliser production.
Liming: immersion in a strong alkaline bath that opens the collagen structure. At this point, there could be a second fleshing step after liming to clean residual flesh (Ozgunay et al., 2007). According to the legislation, limed hides must be treated with lime and in brine at a pH of 12–13 for at least 8 h.
Splitting: the hides are split mechanically into two or three layers.
Deliming/Bating (drenching): These are enzymatic and chemical treatments aimed at opening structures, to remove unwanted proteins and excess of natural fat from the hide and to provide a homogenous structure. In order to remove the lime, hides are subject to neutralisation (i.e. with ammonium or organic salts, enzymes or carbon dioxide) and degreasing (using surfactants or solvents). The hides are bated, i.e. bathing at 38–40°C and a pH of 8.5 for proteolytic enzymes to optimally break down collagen, elastin and reticulin in order to achieve desired consistency.
Pickling: treatment with an acidic solution (pH < 3, sulfuric acid, formic acid or acetic acid) for around 16 h to fully neutralise the alkali. Salt is added to prevent the hide from swelling. Bactericides or fungicides [e.g. 0.05% 2‐(thiocyanomethylthio) benzothiazole;Biosecurity New Zealand, 2007] can also be added.
At the end of all these steps, the obtained product is the pickled pelt that is ready for tanning. The pickling process allows the storage and transport of the pelts for up to 12 months.
Tanning
Tanning is either done with chromium salts, vegetable tannins or organic compounds which cross‐link the fibres stabilising the hide and producing the ‘resistant leather’ as it is known. Tanning is usually done after the hide has been degreased with solvents and surfactants. The final product is dried and dyed (CPRAC, online).
After pickling, chrome tanning consists of the addition of chromium salts (Cr3+) to a pickled pelt that has a very low pH. In order to increase it, an alkaline buffer is added and the chromium ions cross‐link with free carboxyl groups of the collagen making the hide resistant to bacteria and high temperature. The product resulting from this treatment is called ‘wet blue’ and contains 40% of dry matter and 2–3% of dry weight of Cr3+.
Post‐tanning
The next steps of the processing include re‐tanning using one or more combined tanning agents and dying with the desired colour and treatment with fat to increase the smoothness of the final product. Then, excess water and fat are removed and the final product dried (sammying), usually with vacuum drying. Further trimming of unwanted parts can be carried out at this stage.
Finishing
The final step of the full process is the application of multiple coats on the surface of the leather to improve resistance and aspect. Further trimming of unwanted parts can also be carried out at this stage.
3.2.5. Wool and hair
As already mentioned in Section 3.1, point 3, Article 10 of Regulation (EC) 1069/2009 defines as Category 3 material ABP: ‘wool and hair’ originating from (h) live animals that did not show any signs of diseases communicable to human or animals; (n) dead animals that did not show any signs of disease communicable through that product to humans or animals, other than those referred to in point (b).
The animal fibre ‘wool’ of the pelage is a keratin protein‐based product formed in specialised hair follicles located in the skin of a range of animal species and breeds farmed in Europe. Its use in recent decades has been affected by issues such as political changes (e.g. the democratisation of eastern Europe) and the economic integration of the European community, poor economic returns and by competition from petrol‐carbon or oil‐based artificial fibres. Ecologically sensitive methods for wool production and processing and its inherent degradability have recognised importance. Primary hair follicles typically, although with some exceptions, produce outer fibres of greater diameter, than the underlying and more valuable finer fibres from secondary follicles. The fibres from different animal species have various properties, such as colour, medullation, tensile strength, diameter (fineness) and staple length. These properties determine the end use, from small diameter superfine garments to the coarser fibres utilised in carpets and furniture upholstery (Galbraith, 2019).
‘Hair’ fibre is produced by the division of keratinocyte (epidermal) cells which line the base of the follicle in contact with underlying dermal tissue (Galbraith, 2010a,b). These cells divide and migrate towards, and beyond, the skin surface. The rate of proliferation of these cells also determines the rate of growth of fibre (cortex and cuticle), and fleece, and determines staple length. As the cells migrate, they deposit a range of proteins in the internal skeleton and other proteins and lipids which are important in adhesion between cells and which contribute to the properties of softness, flexibility, moisture absorption and tensile strength (Lyons, 2009). In addition, consistency of husbandry and nutrition of animals have implications for uniformity of chemical and physical composition along the length of fibres (Galbraith, 2000).
The end use of wool and hair is determined by their physical properties and the preferences of consumers. They can be used in clothing (typically smallest diameters and highest monetary value for knitwear and suiting), in domestic ‘home’ environment (typically coarser and hard wearing for upholstery, carpets, insulation) and external environment [materials in transport vehicles (carpets, upholstery)], horticulture (plant beddings, fertilisers) or, if of inadequate quality, unused and deposited in land fill or incinerated (Galbraith, 2019).
Different methods to treat wool and hair as raw materials are reported in the literature. The first step is represented by degreasing using different solutions such as ammonia, anhydrous sodium carbonate and ethoxylated alkyl non‐ionic detergent (Berechet et al., 2018). The washing/degreasing step consists of the immersion of the wool and hair in baths of water, soap and different solutions. Little data are reported in the literature on the washing step procedures currently applied. Berechet et al. (2018) described ammonia solution 25% p.a., anhydrous sodium carbonate p.a. and ethoxylated alkyl non‐ionic detergent as possible solutions for wool degreasing. The second step is enzymatic hydrolysis or hydrolysis by acids (HCl, HCOOH, H2SO4) or bases (NaOH, KOH and CaO). The process is performed at high temperature injecting heated or superheated water (120° for 20 min or 150–170°C for 1 h) into the hydrolysis reactor to reach the desired physical conditions (Onifade et al., 1998; Sargison, 2009; Bhavsar et al., 2017). The alkaline or acid hydrolysis leads to the degradation of proteins to obtain oligopeptides and amino acids. These treatments also break disulfide bonds characteristic of wool which confers specific mechanical properties to this fibre (Gupta and Ramnani, 2006).
The application of acid and alkaline hydrolysis at a commercial scale has its limitations, such as the cost of the chemicals and purification of the final product, etc., while enzymatic hydrolysis requires a high capital investment and is a time‐consuming process. Hydrolysis with superheated water is a process in which the extraction of proteins from the wool matrix is carried out under controlled conditions. The high temperature of the hydrolysis treatment sterilises the final product, which indirectly avoids potential health‐related problems ahead of the final application of the product obtained (Liu et al., 2014).
Nustorova et al. (2006) described the preparation of wool hydrolysate using a thermo‐chemical method previously described by Gousterova et al. (2003), experimentally represented by alkaline hydrolysis in an autoclave. A defined quantity of wool waste (10 g) was mixed with 100 mL of 0.15 mol/L KOH–0.05 mol/L NaOH and heated at 120°C for 20 min. The supernatant fluid (after centrifugation at 4,000g for 20 min) was concentrated on a rotary vacuum evaporator and dried at 70°C, ground to powder and stored in a tightly stoppered flask.
Bhavsar et al. (2017) tested superheated water hydrolysis of waste wool. In the process, waste wool was treated with saturated steam at a temperature of 170°C and pressure of 7.0 bar for 60 min.
Composting of wool scouring sludge was tested in the United Kingdom (Pearson et al., 2003). Due to the high moisture content and small particles in the scouring sludge, mixing with green waste, as additional compostable material, was necessary. In particular, 4.5 tonnes of wool scouring sludge were thoroughly mixed with 3.4 tonnes of green waste. The composting pile was aerated through a pre‐installed air distributor. After 110 days, all the parameters showed that composting was an appropriate and useful process for wool scouring sludge waste decomposition to avoid pollution.
Composted waste wool or hydrolysates prepared with microbial or enzymatic pretreatments show promising results in field experiments as fertilisers, and also as substrates for biogas production. There are already some innovative ideas described for waste wool applications (production of wool peptone, amino acids, keratinolytic enzymes), but further research may reveal even more possibilities for high value‐added product development from waste wool.
3.2.6. Feathers and down
Article 10 of Regulation (EC) 1069/2009, point 3, defines ‘feather’ as Category 3 ABP originating from: ‘(b) animals that have been slaughtered in a slaughterhouse and considered fit for human consumption following the ante‐mortem inspection or game killed for human consumption in accordance with Community legislation; (h) live animals that did not show any signs of diseases communicable through that product to human or animals; (n) dead animals that did not show any sign of disease communicable to humans or animals.’
The poultry industry has become one of the largest food industries in the world, producing large quantities of feather waste. Between 5% and 10% of the total weight of a chicken is made of feathers (Callegaro et al., 2019). More than 1 million metric tonnes of feathers are produced annually as a by‐product at European poultry slaughterhouses (Goerner‐Hu et al., 2020). Due to a large variety of chemical hazards and microbiota present on the feathers, including pathogens, they must be treated quickly. Poultry feathers are rich in keratin protein, which makes them a good source of nitrogen fertiliser (Joardar and Rahman, 2018).
Chicken feather waste can be:
Incinerated. This process is effective at inactivating biological hazards but requires a high energy consumption (Saidan et al., 2017) and produces large amounts of carbon dioxide.
Composted with manure. The composting process is slow and subject to the special requirements of veterinary inspection and requires a closed composting area with a sewage carry system, and periodic microbiological tests according to Commission Regulation (EU) No 142/2011. A problem for composting is odorous emission of hydrogen sulfide that persists in the air for a long period. Moreover, according to Tronina and Bubel (2008), composting may not fully inactivate pathogenic microorganisms.
Hydrolysed (Tesfaye et al., 2017a,b). Feather hydrolysis provides valuable amino acids, proteins and peptides in the mixture with acylglycerols and higher fatty acids. Chemical hydrolysis leads to destruction of the native structure of keratin and the feather waste becomes more water soluble. Acidic hydrolysis is highly efficient but causes loss of some amino acids. Alkaline hydrolysis is slower and can be incomplete, but the loss of amino acids is lower. The yield of the hydrolytic processes depends on pH, temperature and reaction time, and also on the type and concentration of acid or base used. As a drawback, commonly applied hydrolysis leads to the requirement for subsequent recycling of the process solutions, including neutralisation and elimination of undesirable salts (Solcova et al., 2021).
Treated in dimethyl sulfoxide or other solvents to get value added products from feather keratin, generated in excess from various livestock industries (Azmi et al., 2018).
3.2.7. Pig bristles
Article 10 of Regulation (EC) 1069/2009, point 3 (b), defines ‘pig bristles’ as Category 3 ABP originating either from animals that: ‘have been slaughtered in a slaughterhouse and considered fit for human consumption following the ante‐mortem inspection […] or game killed for human consumption in accordance with Community legislation.’
Pig bristles consist primarily of keratin (90% or more), an insoluble protein packed with fibres cross‐linked by disulfide bonds. The slaughterhouses collect both bristles and hooves during or shortly after the dehairing process. The weight of the pig bristles harvested from a single pig could reach 0.9 kg (Gonzalo et al., 2016). Considering that approximately 245 million pigs were slaughtered in the EU in 2019,24 the total throughput of this by‐product would be 220,000 tonnes of pig bristles annually.
Preparation of the pig bristles for further treatment is based on soaking in warm water and soap for 60 min before rinsing and drying at 60°C overnight (Gonzalo et al., 2016). The traditional technology for degradation of raw pig bristles is based on long‐term heating, alkaline hydrolysis or the hydrolysation with high pressure and heat (6 bar, 150°C for at least 20 min) (Gonzalo et al., 2016; Falco et al., 2019; Espersen et al., 2020). Other treatments were described in individual studies, e.g. thermo‐chemical treatment (121°C, 20 min, 1 g bristle per 100 cm3 sodium sulfite solution) (Łaba et al., 2016), or washing and degreasing with a methanol‐chloroform solution (Laba and Rodziewicz, 2014), among others. The main purpose of these treatments is the extraction of pure keratin and its subsequent conversion into smaller protein molecules of higher nutritional or industrial value through enzymatic, chemical or microbial based techniques.
3.2.8. Horns, horn products, hooves and hoof products
Point 3 Article 10 of Regulation (EC) 1069/2009 defines as Category 3 material ABP horns, and hoof cuts originating from: ‘(b) horns of animals, other than ruminants requiring TSE testing, and ruminants which have been tested with a negative result in accordance with Article 6(1) of Regulation (EC) No 999/2001; (h) live animals that did not show any signs of disease communicable through that product to humans or animals; (n) hooves and horns originating from dead animals that did not show any signs of disease communicable through that product to humans or animals, other than those referred to in point (b) of this Article.’
Horns and hooves are by‐products in slaughterhouses and meat plants. Horns vary in size, shape, colour and curvature according to the breed, age, sex, etc. The term horn in everyday language is commonly applied to both the horn pith, the inner part, and the horn itself, and these are used for different purposes. The horn pith is also called horn core and is similar to bone, although it contains more ossein. As a result, it is a valuable raw material for gelatine production. Alternatively, it may be used for the production of bone meal. Horns and hooves are keratin‐rich (α‐keratin) materials consisting of tightly packed protein chains in α‐helices stabilised by high degrees of disulfide and hydrogen bond cross‐linking, as well as hydrophobic interactions, which render them insoluble and resistant to biodegradation. This is a major obstacle in native keratin processing. Animal remains rich in α‐keratin are in nature relatively quickly biodegraded by keratinolytic microorganisms (Korniłłowicz‐Kowalska and Bohacz, 2011). They can be co‐digested together with swine manure or slaughterhouse sludge in an anaerobic digester at 25°C, without physico‐chemical or enzymatic treatment, to generate methane (Xia et al., 2015). The resulting nutrient‐rich digestate may be used in agriculture (Salminen and Rintala, 2002). Hooves can also be treated using urea (to break non‐covalent bonds), sodium dodecyl sulfate (for disruption of strong intermolecular interactions) and mercaptoethanol (to cleave the disulfide bonds in keratin), at 60°C, to get keratin in aqueous solution first and a lyophilised form afterwards (Shen et al., 2020).
Hoof and horn meals contain from 16% to 17% nitrogen and are specifically used as manure in tea and coffee plantations.
The horns and hooves are treated separately. After the animal is slaughtered, the horns are cut off with a saw or a cleaver or shears at their base. The horn pith can be removed by steaming for a few moments or by immersing the horn in hot water at 65.6°C (150°F). After this, a blow from a hammer will separate the pith from the horn.
The hooves are soaked in water until they become spongy and can be freed from the bones, after which they are dried at the sun (Omole and Ogbiye, 2013) or, according to producers’ sites, at 140°C. The horn and hooves of cattle are steamed under pressure, digested, crushed and disintegrated for preparation of hoof and horn meal by 8 hours rendering and fine milling. The hoof and horn meal may be mixed with bone meal and used as fertiliser because of the high nitrogen content.
3.3. Standard or alternative processing methods for the list of materials included in the mandate
In order to provide an answer to AQ1, Commission Regulation (EU) 142/2011 was thoroughly reviewed to extract information on the processing standards for the declaration of the end points in the manufacturing chain of the ABP of relevance for the mandate (hides and skins, wool and hair, feather and down, pig bristles, horns and horn products, hooves and hoof products) or the standard or alternative methods approved for the production of derived products of relevance for the mandate (ash, glycerine and other materials derived from the production of biodiesel and renewable fuels), as well as any other relevant information detailed in that EU regulation. The extracted information is summarised in Table 1.
Table 1.
Relevant extracts of the Commission Regulation (EU) 142/2011 for the list of materials included in the mandate
| Group | Raw material | Pretreatment | Transformation processes for the declaration of the end points and standard or alternative methods for the production of derived products | Derived products, derogations and other provisions | Com. Reg. (EU) 142/2011 |
|---|---|---|---|---|---|
| 1) Ash derived from incineration, co‐incineration and combustion | Category 2 Category 3 | Incineration or co‐incineration plants shall be designed, equipped, built and operated in such a way that the gas resulting from the process is raised in a controlled and homogeneous fashion, even under the most unfavourable conditions, to a temperature of 850°C for at least 2 s. | Slag and bottom ashes total organic carbon content is less than 3% or their loss on ignition is less than 5% of the dry weight of the material. | Annex III, Chapter 1, Section 2 Annex III, Chapter 2, Section 1c | |
| Category 2 Category 3 | Incineration or co‐incineration plants shall be designed, equipped, built and operated in such a way that the gas resulting from the process is raised in a controlled and homogeneous fashion, even under the most unfavourable conditions, to a temperature of 1,100°C for 0.2 s. | Slag and bottom ashes total organic carbon content is less than 3% or their loss on ignition is less than 5% of the dry weight of the material. | Annex III, Chapter 1, Section 2 Annex III, Chapter 2, Section 1c | ||
| 2) Glycerine derived from the production of biodiesel and renewable fuels | Fat fraction derived from ABP of all categories | Processing method 1 for Category 1 and Category 2 Processing methods 1–5 or 7 for Category 3 Material derived from fish: processing methods 1–7 (Details of the methods 1–7 are displayed in Appendix B) | D. Biodiesel production process: Insoluble impurities < 0.15% by weight. Esterification is not required for processed fat derived from Category 3 material. Esterification at pH < 1 by adding sulfuric acid (H2SO4) or an equivalent acid and the mixture must be heated to 72°C for at least 2 h during which it must be intensely mixed. Transesterification must be carried out by increasing the pH to about 14 with potassium hydroxide or with an equivalent base at 35–50°C for at least 15 min. Transesterification shall be carried out twice under the conditions described in this point using a new base solution. This process must be followed by refinement of the products including vacuum distillation at 150°C, leading to biodiesel. A process using equivalent process parameters authorised by the competent authority. | The biodiesel production process may be: i) in the case of biodiesel and of residues from the distillation of biodiesel, used as a fuel without restrictions under this Regulation (end point); ii) in the case of potassium sulfate, used for direct application to land or for the production of derived products for application to land; iii) in the case of glycerine derived from Categories 1 and 2 material which has been processed in accordance with processing method 1 as set out in Chapter III: — used for technical purposes, — transformed into biogas, in which case the digestion residues may be applied to land within the national territory of the producing Member State, subject to the decision of the competent authority, or — used for denitrification in a wastewater treatment plant, in which case the residues of the denitrification may be applied to land in accordance with Council Directive 91/271/EECa; iv) in the case of glycerine derived from Category 3 material: — used for technical purposes, — transformed into biogas, in which case the digestion residues may be applied to land, or — used for feeding, provided that the glycerine is not derived from Category 3 material referred to in Article 10(n), (o) and (p) of Regulation (EC) No 1069/2009; Any waste other than animal by‐products and derived products provided for in point 2, resulting from the processing of animal by‐products in accordance with this Section, such as sludge, filter contents, ash and digestion residues, shall be disposed of in accordance with Regulation (EC) No 1069/2009 and with this Regulation. | Annex IV, Chapter IV, Section 2D Annex IV, Chapter IV, Section 3 Point 2b |
| 3) Other materials derived from the production of biodiesel and renewable fuels | i) Processed rendered fats derived from Category 2 material ii) Fish oil or rendered fats derived from Category 3 material, processed iii) Fish oil or rendered fat which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No. 853/2004, respectively | Processing method 1 for Category 2 Fish oil or rendered fats from Category 3 processed using: — any of the processing methods 1–5 or processing method 7; or — in the case of material derived from fish oil, any of the processing methods 1–7; | J. Multi‐step catalytic process for the production of renewable fuels (use of rendered fats derived from Category 1 material for this process shall be prohibited) Pretreatment: (i) the bleaching of the centrifuged materials by passing them through a clay filter; (ii) the removal of remaining insoluble impurities by filtration. Multi‐step catalytic process which consists of a hydro‐deoxygenisation step, followed by an isomerisation step. The materials must be submitted to a pressure of at least 20 bar at a temperature of at least 250°C for at least 20 min. | c) the multi‐step catalytic process for the production of renewable fuels may be: i) in the case of gasoline and the other fuels resulting from the process, used as a fuel without restrictions under this Regulation (end point); ii) in the case of used clay from bleaching and sludge from the pretreatment process referred to in point J(2)a of Section 2: — disposed of by incineration or co‐incineration, — transformed into biogas, — composted or used for the manufacture of derived products referred to in Article 36(a)(i) of Regulation (EC) No 1069/2009; | Annex IV, Chapter IV, Section 2J Annex IV, Chapter IV, Section 3 Point 2c |
| i) Processed rendered fats derived from Category 1 ii) Fish oil or rendered fats derived from Category 3 material, processed iii) Fish oil or rendered fat which have been produced in accordance with Sections VIII or XII of Annex III to Regulation (EC) No. 853/2004, respectively | Processing method 1 for Category 2 Fish oil or rendered fats from Category 3 processed using: — any of the processing methods 1–5 or processing method 7; or — in the case of material derived from fish oil, any of the processing methods 1–7 | L. Multiple‐step catalytic hydro‐treatment for the production of renewable fuels Pretreatment which consists at least of bleaching of the starting material, including rendered fats, with acid in the presence of bleaching clay and subsequent removal of the used bleaching clay and insoluble impurities by filtration. Prior to this treatment, rendered fat may be degummed with an acid and/or caustic solution in order to remove impurities from the rendered fat by forming gums and subsequently separating those gums by centrifugation. The pretreated materials must be submitted to a hydro‐treatment process which consists of a catalytic hydro‐treatment step, a stripping step followed by an isomerisation step. The materials must be submitted to a pressure of at least 30 bar at a temperature of at least 265°C for at least 20 min. | f) the multiple‐step catalytic hydro‐treatment for the production of renewable fuels may be: i) in the case of renewable diesel, renewable jet fuel, renewable propane and renewable gasoline resulting from the process, used as a fuel without restrictions under this Regulation (end point); ii) in the case of gum sludge and used bleaching clay from the pretreatment process referred to in point L(2)a of Section 2: — disposed of in accordance with Article 12a or (b) of Regulation (EC) No. 1069/2009, — disposed of by burial in an authorised landfill, — transformed into biogas, provided the digestion residues from the biogas transformation are disposed of by incineration, co‐incineration or burial in an authorised landfill, — used for technical purposes referred to in Article 36(a)(i) of Regulation (EC) No. 1069/2009. | Annex IV, Chapter IV, Section 2L Annex IV, Chapter IV, Section 3 Point 2f | |
| 4) Hides and skins | B. Untreated hides and skins may be placed on the market subject to the health conditions applicable to fresh meat pursuant to Directive 2002/99/EC. | C. End point for hides and skins 1) Hides and skins of ungulates which pursuant to the decision of an operator are destined for purposes other than human consumption, and which comply with the requirements of Regulation (EC) No. 853/2004 for raw materials for gelatine or collagen intended for use in food may be placed on the market without restrictions in accordance with this Regulation. 2) The following treated hides and skins may be placed on the market without restrictions in accordance with this Regulation: a hides and skins having undergone the complete process of tanning; (b) ‘wet blue’; (c) ‘pickled pelts’; (d) limed hides (treated with lime and in brine at a pH of 12 to 13 for at least eight hours). | 3) By way of derogation from point C.2, the competent authority may require that consignments of treated hides and skins referred to in point 2(c) and (d) are accompanied by a commercial document in accordance with the model set out under point 6 of Chapter III of Annex VIII, when they are supplied to establishments or plants producing petfood, organic fertilisers or soil improvers or transforming those materials into biogas | Annex XIII, Chapter V, Point B Point C | |
| 5) Wool and hair | Untreated wool, untreated hair, untreated pig bristles and untreated feathers, parts of feathers and down must be Category 3 materials referred to in Article 10(b) (iii), (iv) and (v) and Article 10(h) and (n) of Regulation (EC) No. 1069/2009. They must be securely enclosed in packaging and dry | B. End point for wool and hair: Factory‐washed wool and hair, and wool and hair which has been treated by another method which ensures that no unacceptable risks remain, may be placed on the market without restrictions in accordance with this Regulation. Member States may authorise the placing on the market of untreated wool and hair from farms or from establishments or plants which have been registered in accordance with Article 23 of Regulation (EC) No. 1069/2009 or approved in accordance with Article 24(1)(i) of the same Regulation on their territory without restrictions in accordance with this Regulation, if they are satisfied that no unacceptable risks to public and animal health arise from the wool and from the hair. Wool and hair produced from animals other than those of the porcine species may be placed on the market without restrictions in accordance with this Regulation, provided: a) it has undergone factory washing which consists of the immersion of the wool and hair in series of baths of water, soap and sodium hydroxide or potassium hydroxide; or b) it is dispatched directly to a plant producing derived products from wool or hair for the textile industry and such wool or hair has undergone at least one of the following treatments: i) chemical depilation by means of slaked lime or sodium sulfide; ii) fumigation in formaldehyde in a hermetically sealed chamber for at least 24 h; iii) industrial scouring which consists of the immersion of wool and hair in a water‐soluble detergent held at 60–70°C; iv) storage, which may include the journey time, at 37°C for 8 days, 18°C for 28 days or 4°C for 120 days | Movements of pig bristles and wool and hair of animals of the porcine species from regions in which African swine fever is endemic shall be prohibited except for pig bristles and wool and hair of animals of the porcine species that have: a) been boiled, dyed or bleached; or b) undergone some other form of treatment which is certain to kill pathogenic agents, provided that evidence to this effect is submitted in the form of a certificate from the veterinarian responsible for the place of origin. Factory washing may not be regarded as a form of treatment for the purposes of this provision | Annex XIII, Chapter VII, Point B Point A.2 | |
| 6) Feathers and down | Untreated wool, untreated hair, untreated pig bristles and untreated feathers, parts of feathers and down must be Category 3 materials referred to in Article 10(b) (iii), (iv) and (v) and Article 10(h) and (n) of Regulation (EC) No. 1069/2009. They must be securely enclosed in packaging and dry | C. End point for feathers and down Feathers, parts of feathers and down which have been factory‐washed and treated with hot steam at 100°C for at least 30 min may be placed on the market without restrictions in accordance with this Regulation. | However, in the case of untreated feathers, parts of feathers and down sent directly from the slaughterhouse to the processing plant, the competent authority may allow a derogation from the requirement to dry materials transported on its territory, provided that: a all necessary measures are taken to avoid any possible spread of disease; (b) the transport takes place in waterproof containers and/or vehicles which must be cleaned and disinfected immediately after each use. 3. The provisions of point 1 shall not apply to decorative feathers or feathers: a carried by travellers for their private use; or (b) in the form of consignments sent to private individuals for non‐industrial purposes | Annex XIII, Chapter VII, Point A.1 Point C | |
| 7) Pig bristles | Untreated wool, untreated hair, untreated pig bristles and untreated feathers, parts of feathers and down must be Category 3 materials referred to in Article 10(b) (iii), (iv) and (v) and Article 10(h) and (n) of Regulation (EC) No. 1069/2009. They must be securely enclosed in packaging and kept dry | No end point for pig bristles is reported in Commission Regulation (EU) 142/2011. | Movements of pig bristles and wool and hair of animals of the porcine species from regions in which African swine fever is endemic shall be prohibited except for pig bristles and wool and hair of animals of the porcine species that have: a been boiled, dyed or bleached; or (b) undergone some other form of treatment which is certain to kill pathogenic agents, provided that evidence to this effect is submitted in the form of a certificate from the veterinarian responsible for the place of origin. Factory washing may not be regarded as a form of treatment for the purposes of this provision | Annex XIII, Chapter VII, Point A.1 Point A.2 | |
| 8) Horns, horn products, hooves and hoof products | The placing on the market of horns and horn products, excluding horn meal, and hooves and hoof products, excluding hoof meal, intended for the production of organic fertilisers or soil improvers shall be subject to the following conditions: a) they must originate from animals that: (i) either have been slaughtered in a slaughterhouse, after undergoing an ante‐mortem inspection, and were found fit, as a result of such inspection, for slaughter for human consumption in accordance with Union legislation; or (ii) did not show clinical signs of any disease communicable through that product to humans or animals; b) they must have undergone a heat treatment for 1 hour at a core temperature of at least 80°C; c) the horns must be removed without opening the cranial cavity. c) the horns must be removed without opening the cranial cavity. d) at any stage of processing, storage or transport, every precaution shall be taken to avoid cross‐contamination. e) they shall be packed either in new packaging or containers; or transported in vehicles or bulk containers which have been disinfected prior to loading using a product approved by the competent authority. f) the packaging or containers must: i) indicate the type of product (such as horns, horn products, hooves or hoof products); ii) be marked with the name and address of the approved or registered establishment or plant of destination. | Annex XIII Chapter XII |
Council Directive 91/271/EEC of 21 May 1991 concerning urban waste‐water treatment, OJ L 135, 30.5.1991, p. 40.
3.4. Indicator microorganisms
The behaviour of microorganisms (bacterial, fungi and viruses) throughout processing or transformation methods can be difficult (maybe even impossible) to elucidate for every single individual organism that may represent a hazard, in part due to their irregular distribution and usually low occurrence and concentration in raw materials that preclude a robust quantification of inactivation levels. In such instances, indicator microorganisms have been used. For inactivation or heat treatment processes, indicator microorganisms typically represent the most resilient or resistant organisms within specific categories. The effect of processing or transformation methodologies can therefore be assessed, as if these resilient indicator microorganisms are inactivated, then less resilient biological hazards can also be assumed to be inactivated. Thus, indicator microorganisms are typically chosen to represent proxies for less resilient/stable organisms. A list of common indicator microorganisms is given in Table 2.
Table 2.
Indicator microorganisms frequently used as proxies for less stable organisms
| Name | Indicator for |
|---|---|
| Escherichia coli | Gram −ve, non‐spore‐forming coliform bacteria |
| Salmonella Senftenberg | Gram −ve, non‐spore‐forming bacteria |
| Enterococcus faecalis | Gram +ve, non‐spore‐forming bacteria |
| Clostridium spp. | Gram +ve, spore‐forming bacteria |
| Mycobacterium spp. | Acid‐fast, thermoresistant bacteria |
| Bovine parvovirus | Viruses |
| Calicivirus | Viruses |
| Ascaris sp. | Parasites |
| Cryptosporidium parvum | Parasites |
Data on inactivation of indicator microorganisms on industrial‐scale systems are generally recommended to determine the inactivation efficiency of a process. Ideally, the performance and validity of an indicator should be established for each selected inactivation process and matrix of concern. However, some indicator organisms are widely recognised as valuable for such process validation tests. In the particular case of the indicator organisms mentioned in the requirements for alternative transformation parameters for biogas and composting plants in terms of the validation of the intended process, referred to in point 1 of Section 2 of Chapter III of Annex V Commission Regulation (EU) No 142/2011, these are Enterococcus faecalis, Salmonella Senftenberg (775W, H2S negative), parvovirus and eggs of Ascaris sp.
3.4.1. Enterococcus faecalis
E. faecalis is a member of the genus Enterococcus and is a Gram‐positive non‐spore‐forming bacterium. It is described as an opportunistic pathogen which particularly affects immunocompromised populations. E. faecalis is found in the gut of healthy humans but only reported in some warm‐blooded animals, including dogs, and chickens (Pourcher et al., 1991; Wheeler et al., 2002). E. faecalis is identified as a heat‐resistant organism, resulting in its successful application in process validation (Watcharasukarn et al., 2009). Indeed, E. faecalis often serves also as an indicator microorganism to characterise the performance of hygienisation processes (Sahlström, 2003). Another enterococcus, E. faecium, is also widely evaluated as an indicator organism for validating bacterial inactivation in different kinds of thermal processes (Kopit et al., 2014; Ceylan and Bautista, 2015). However, it is E. faecalis the indicator organism which is mentioned in point 1 of Section 2 of Chapter III of Annex V of Commission Regulation (EU) No 142/2011. Both E. faecalis and E. faecium serve as indicator microorganisms for both Gram‐positive and Gram‐negative non‐spore‐forming bacteria, given the higher thermal tolerance that Gram‐positive cocci generally show as compared with that of other non‐spore‐forming bacterial species.
3.4.2. Salmonella Senftenberg
Salmonellae are Gram‐negative non‐spore‐forming motile rod bacteria. They are widespread in nature and found in food, soil, water, manure (Winfield and Groisman, 2003) and biological waste streams (Burtscher and Wuertz, 2003). The main reservoir of non‐typhoidal Salmonella are the animals, but they are well adapted to their surroundings and cycle between environmental matrices and living hosts. Certain serovars or strains of Salmonella enterica are noted for their high resistance to thermal treatments, relative to other Salmonella spp. or Gram‐negative bacteria, the most prominent being Salmonella Senftenberg, particularly the strain 775W (Ng et al., 1969). In different model systems, this strain has shown D‐values (times needed to reduce the bacterial population at a given temperature by 1 log10 unit) around 10‐fold to 20‐fold higher than those of other serovars, such as Salmonella Typhimurium or Salmonella Enteritidis (Doyle and Mazzotta, 2000). Salmonella Senftenberg is not a major food‐borne pathogen, and it is often used as an indicator organism to validate thermal treatments (Ng et al., 1969). The implication is that if a particular thermal process achieves a sufficient level of reduction for S. Senftenberg 775W, it will also be effective against all salmonellae and other Gram‐negative non‐spore‐forming bacteria (Doyle and Mazzotta, 2000).
3.4.3. Parvovirus
Parvovirus is a relatively common thermoresistant virus found in livestock (Lund et al., 1996) and humans (Qiu et al., 2017). Animal parvoviruses have been reported to be the most heat‐resistant viruses (Sauerbrei and Wutzler, 2009), and are therefore frequently used as indicators to validate the virucidal efficacy of thermal processes. In a systematic review conducted by Nims and Plavsic (2013a) comparing different viral families for their susceptibility to heat inactivation, it was demonstrated that, among the four families included in the ELS for inactivation data, Parvoviridae is by far the most heat‐resistant viral family followed by Caliciviridae and Picornaviridae. Likewise, Knight et al. (2013) reviewed available data and mechanisms regarding the thermal inactivation of a number of important pathogenic animal viruses (e.g. African swine fever virus, classical swine fever virus, infectious bursal disease virus, Rift Valley fever virus, avian influenza virus, Newcastle disease virus, Foot and mouth disease virus, swine vesicular disease virus, Bluetongue virus) in comparison with relevant indicator viruses and concluded that non‐enveloped small DNA viruses, such as parvovirus, were amongst the most heat‐resistant viruses reported.
Among parvoviruses, Porcine parvovirus and Bovine parvovirus have been suggested as good indicator viruses because of their relatively high thermal resistance, because they appear commonly in livestock (Srivastava and Lund, 1980) and because in vitro culture systems are available to test their viability. Given their persistence at high temperatures, they are regularly used as indicator viruses for thermal treatments validation. In addition, due to their relatively small size (20–24 nm diameter), screening or filtration systems that remove parvoviruses would typically be expected to remove other larger viruses, and hence, they are also used as indicators to evaluate processes for the production of biological materials from cell cultures in the pharma and biotechnology industries (Stuckey et al., 2014). Bovine parvovirus strain Haden has been specifically recommended for use in the evaluation of thermo‐chemical and thermal disinfection procedures to assess their virucidal effectiveness (Bräuniger et al., 1994, 2000).
3.4.4. Ascaris spp.
Ascaris spp. are parasites and members of the geohelminths, which can colonise the intestinal tract of animals and humans. The eggs are highly resistant to adverse environmental conditions, including desiccation and chemical treatment (including acids) (Pecson and Nelson, 2005). The eggs of Ascaris suum, a close relative of the very similar human‐infecting species Ascaris lumbricoides, have been used as an indicator for helminths when examining the effects of various waste treatment processes (USEPA, 1999), such as aerobic (Kato et al., 2003) and thermophilic anaerobic digestion (Aitken et al., 2005), ammonia treatment of wastewater (Ghiglietti et al., 1997) or composting of sewage sludge (Paluszak et al., 2003). Ascaris suum is a parasitic helminth of pigs, which occasionally infects cattle and on rare occasions humans, and is considered one of the most resilient helminths (USEPA, 1999). Ascaris eggs are also one of the most heat‐resistant parasitic ova and hence are well suited as an indicator of parasite survival in general (Sahlström et al., 2008).
3.5. Viral hazard identification in Groups 4, 5, 6, 7 and 8 (AQ3)
In order to identify the viral hazards to address in the Category 2 and 3 materials and derived products of the mandate, there was a need to differentiate between intrinsic risk (i.e. risk from hazards isolated in the matrix) and potential contamination risk (i.e. faecal contamination, unhygienic handling and storage) in the selected matrices. Intrinsic viral hazards are most likely to be prevalent in the raw materials and hence of greatest concern given the potential for widespread occurrence in the raw tissues. Contamination events may be sporadic, involve a wide range of viral hazards or be case‐specific, making the inclusion of a particular contamination risk in this assessment very challenging. If a contamination event is identified as likely to occur it should be considered on a case‐by‐case basis. Thus, for the scope of this mandate, only viral hazards that are present in the unprocessed matrices as intrinsic viral hazards were considered.
Groups 1, 2 and 3 (ash derived from incineration, co‐incineration and combustion; glycerine; other products of materials derived from the production of biodiesel and renewable fuels) are derived products. Although they can be obtained using a wide range of raw materials for their production, no intrinsic viral hazards in the final products were identified during the screening process. For these reasons, they were eliminated from the hazard identification assessment and the focus was on Groups 4–8.
Results of the ELS and screening for the viral hazards are given in Tables 3 and 4. The greatest number of hits was found for group 5 (wool and hair) and group 4 (hides and skin), reflecting the presence of intrinsic viral hazards in these matrices, while no intrinsic hazards were identified in group 7 (pig bristles). The occurrence of the family Poxviridae is also reflected in Tables 3 and 4 with the greatest number of hits (predominantly in hides and skin and wool and hair). While many families are specific to a matrix, the family Flaviviridae was found to occur in three of the matrices (hides and skin; wool and hair; feathers and down).
Table 3.
Results of the ELS and screening for the viral hazards
| Group | Group description | Number of hitsa | Yb | D | N |
|---|---|---|---|---|---|
| 4 | Hides and skins | 495 | 84 (35) | 14 (1) | 397 |
| 5 | Wool and hair | 728 | 105 (6) | 41 (3) | 582 |
| 6 | Feathers and down | 624 | 35 (9) | 33 (0) | 556 |
| 7 | Pig bristles | 38 | 3 (1) | 3 (0) | 32 |
| 8 | Horns, horn products, hooves and hoof products | 97 | 5 (1) | 10 (0) | 82 |
Y: Yes (presence of virus in the materials); D: doubtful (not clear from the title and abstract the presence of virus in the materials); N: No (no presence of virus in the materials). In parentheses, the number of selected references for viral hazards following the criteria above.
Non‐enveloped viruses are generally considered more resistant to both thermal and chemical processes than enveloped ones (McDonnell, 2020). For this reason, it was decided to further focus only on identified viral hazards belonging to this group.
The following non‐enveloped viruses were identified as relevant hazards in the different raw materials:
Group 4 (Hides and Skins): Papillomaviridae (Bovine papillomavirus, Cottontail rabbit papillomavirus), Reoviridae (Bluetongue virus), Picornaviridae (Foot and mouth disease virus, Swine vesicular disease virus).
Group 5 (Wool and hair): Picornaviridae (Foot and mouth disease virus), Parvoviridae (Ungulate tetraparvovirus).
Group 6 (Feathers and down): Anelloviridae (Chicken anaemia virus, formerly classified as Circoviridae), Circoviridae (Duck circovirus).
Group 7 (Pig bristles): none.
Group 8 (Horns, horn products, hooves and hoof products): Picornaviridae (Senecavirus A).
The main characteristics of the six families of viruses identified in the hazard identification are:
-
a
Papillomaviridae
Papillomaviruses are non‐enveloped, double‐stranded DNA viruses that can infect mucosal and/or cutaneous epithelia and are largely species specific. Bovine papillomaviruses belong to the Papillomaviridae family, which consists of a large number of small DNA oncogenic viruses infecting the epithelium and mucosa of many animals as well as humans causing benign hyperproliferative lesions or cancers (Bocaneti et al., 2016). The rabbit (Shope) papillomavirus, also called cottontail rabbit papillomavirus, is an oncogenic DNA virus of the Papillomaviridae family that is transmitted by biting arthropods (especially continental rabbit ticks, reduviid bugs and mosquitoes) (Hess and Tater, 2012).
-
b
Picornaviridae
Swine vesicular disease (SVD) is a highly contagious viral disease in pigs. SVD virus (SVDV) is currently classified as a porcine variant of human coxsackievirus B5 (CVB5) (Van Rogenmortel et al., 2000) and a member of the genus Enterovirus in the family Picornaviridae. Natural infections caused by SVDV have only been reported in pigs. SVDV is spread primarily by contact with infected swine or their excretions, or by feeding pigs with unheated meat products contaminated with SVDV. The virus is very stable in the environment (Lin and Kitching, 2000). SVDV causes a vesicular disease in pigs clinically indistinguishable from foot‐and‐mouth disease (genus Aphthovirus) and indistinguishable from vesicular disease caused in pigs by Seneca Valley virus (genus Senecavirus).25
Senecavirus A is the only member of the genus Senecavirus within the family Picornaviridae 27. Clinical SVA infection in pigs presents similar characteristics to other vesicular diseases, but clinical signs and lesions are relatively mild, albeit indistinguishable from other vesicular diseases. Cutaneous lesions are found more frequently on the lips, snout and tongue, and on the feet, affecting the coronary band, interdigital area, dewclaws and hoof pads (Segalés et al., 2016).
Foot and mouth disease virus (FMDV) belongs to the genus Aphthovirus within the family Picornaviridae. It causes foot‐and‐mouth disease (FMD) that is an acute and highly contagious disease, responsible for fever, lameness and vesicular lesions on the feet, tongue and teats. FMDV consists of a single‐stranded, plus‐sense RNA genome of approximately 8,500 bases surrounded by four structural proteins to form an icosahedral capsid (Grubman and Baxt, 2004). FMDV has multiple serotypes and broad host range and is thought to spread mainly from animal to animal by aerosol droplets between animals in close contact. FMD seriously affects the livestock industry and threatens the international trade in animals and animal products (Jamal and Belsham, 2013; Li et al., 2021).
-
c
Parvoviridae
Ungulate tetraparvovirus belongs to Tetraparvovirus (previously proposed as ‘Partetravirus’), that became an established genus in the Parvoviridae family in 2018 (Pan et al., 2019). Members of the family Parvoviridae are small, resistant, non‐enveloped viruses with linear, single‐stranded DNA genomes of 4–6 kb. Viruses in two subfamilies, the Parvovirinae and Densovirinae, are distinguished primarily by their respective ability to infect vertebrates (including humans) vs. invertebrates (Cotmore et al., 2019).
-
d
Anelloviridae
Chicken anaemia virus (CAV) is a 25‐nm, non‐enveloped, icosahedral virus with a very small (2.3 kb), single‐stranded, negative sense, circular DNA genome. It is the only recognised member of the Gyrovirus genus of the Anelloviridae family. It was previously classified as a Circovirus, but important differences in genome organisation led to its reclassification into the new Anelloviridae family. Horizontal transmission of CAV infection is by the faecal‐oral route, possibly by the respiratory route, and through infected feather follicle epithelium. Contaminated litter is a common source of introduction. Vertical transmission may occur when seronegative hens become infected and infection continues during egg laying until adequate levels of neutralising antibodies develop in the hens. CAV is ubiquitous throughout the world in poultry operations; infection of young chickens causes anaemia, decreased weight gain, transient immunosuppression and increased mortality (Fatoba and Adeleke, 2019).
-
e
Circoviridae
Duck circovirus (DuCV) is a small, round, non‐enveloped, single‐stranded DNA virus with a circular genome and, being an immunosuppressive virus, it may increase the pathogenicity of coinfecting agents. The classic symptoms are generally considered to be feather disorders, poor body condition and low weight for age (Liu et al., 2020).
-
f
Reoviridae
Bluetongue virus (BTV) is the aetiological agent of Bluetongue (BT), a non‐contagious vector‐borne viral disease of domestic and wild ruminants. BTV is a segmented double‐stranded (dsRNA) virus belonging to the genus Orbivirus of the family Reoviridae. It is transmitted through the bite of hematophagous midges of the Culicoides genus (Wilson and Mellor, 2009). BTV is widely prevalent in sheep, goats, cattle, camels, deer and antelopes. Clinical presentation ranges from asymptomatic to mild fever, salivation, depression, dyspnoea and even abortion and death, leading to severe economic repercussions for livestock breeding (Gong et al., 2021).
To obtain more information about the thermal and chemical resistance of these viral hazards, a literature search with the predefined search string and the respective viral hazard (five non‐enveloped DNA and four non‐enveloped RNA viruses) was conducted, as described in Annex A.
There was no need to include FMDV in the search because sufficient data were available from the REFRESH study data set (Hayrapetyan et al., 2019).
3.6. Thermal inactivation data
Thermal inactivation data for the relevant biological hazards were retrieved, when possible, from comprehensive review articles, like the one by Doyle and Mazzotta (2000) for S. Senftenberg and the one by Sörqvist (2003) for E. faecalis, or from previous reports, such as the one of the REFRESH study (Hayrapetyan et al., 2019). Additionally, references of experimental studies retrieved through tailored searches performed as described in Section 2 and Annex A were also considered when necessary.
3.6.1. Enterococcus faecalis
For E. faecalis, the literature search provided a review investigating the heat resistance of inter alia E. faecalis, however only in liquid matrices (Sörqvist, 2003). Therefore, it was decided to extract additional D‐values on matrices with different characteristics from the non‐review hits of the literature search. The screening of title and abstract of the 71 references extracted from the search on thermal inactivation of E. faecalis produced a shortlist of four additional papers from which data were extracted: Ugwuanyi et al. (1999), Aguirre et al. (2009), Harris et al. (2012) and Saucier and Plamondon (2011). The data from these different sources were consolidated and used to extract D‐values at different heating temperatures and estimate the times needed to inactivate 5 log10 units as a function of the treatment temperature (Figure 4). Details of the data points used to produce Figure 4 are displayed in Table A.1 of Appendix A.
Figure 4.
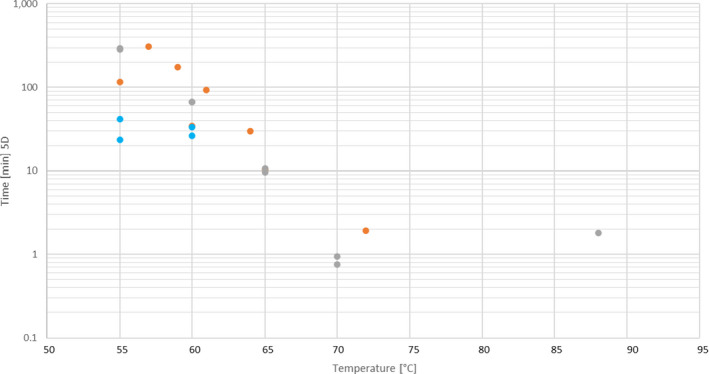
- Orange: liquid matrix; grey: solid matrix; blue: semi‐liquid matrix.
Table A.1.
Details of the data points used to produce Figure 4 from the references identified in the literature review for Enterococcus faecalis
| Hazard | Product group | Product/or medium | Treatment | T (°C) | D (min) | 5D (min) | Reference |
|---|---|---|---|---|---|---|---|
| Enterococcus faecalis | Liquids | Mixed | Heat | 55 | 23.22 | 116.1 | Sörqvist (2003) |
| Enterococcus faecalis | Liquids | Mixed | Heat | 60 | 6.92 | 34.56 | |
| Enterococcus faecalis | Liquids | Mixed | Heat | 65 | 2.05 | 10.25 | |
| Enterococcus faecalis | Liquids | Mixed | Heat | 72 | 0.38 | 1.9 | |
| Enterococcus faecalis | Liquids | Whole milk | Heat | 57 | 61.73 | 308.65 | Aguirre et al. (2009) |
| Enterococcus faecalis | Liquids | Whole milk | Heat | 59 | 34.84 | 174.2 | |
| Enterococcus faecalis | Liquids | Whole milk | Heat | 61 | 18.48 | 92.4 | |
| Enterococcus faecalis | Liquids | Whole milk | Heat | 64 | 5.91 | 29.55 | |
| Enterococcus faecalis | Solid product | Whole raw almond kernels | Hot water | 88 | 0.36 | 1.8 | Harris et al. (2012) |
| Enterococcus faecalis | Solid product | Growth in BHI and treatment in aseptically prepared ground beef | Heating in water bath | 55 | 57.53 | 287.65 | Saucier and Plamondon (2011) |
| Enterococcus faecalis | Solid product | Growth in BHI and treatment in aseptically prepared ground beef | Heating in water bath | 60 | 13.37 | 66.85 | |
| Enterococcus faecalis | Solid product | Growth in BHI and treatment in aseptically prepared ground beef | Heating in water bath | 65 | 1.93 | 9.65 | |
| Enterococcus faecalis | Solid product | Growth in BHI and treatment in aseptically prepared ground beef | Heating in water bath | 70 | 0.19 | 0.95 | |
| Enterococcus faecalis | Solid product | Growth in ME2 and treatment in aseptically prepared ground beef | Heating in water bath | 55 | 58.65 | 293.25 | |
| Enterococcus faecalis | Solid product | Growth in ME2 and treatment in aseptically prepared ground beef | Heating in water bath | 60 | 13.37 | 66.85 | |
| Enterococcus faecalis | Solid product | Growth in ME2 and treatment in aseptically prepared ground beef | Heating in water bath | 65 | 2.12 | 10.6 | |
| Enterococcus faecalis | Solid product | Growth in ME2 and treatment in aseptically prepared ground beef | Heating in water bath | 70 | 0.15 | 0.75 | |
| Enterococcus faecalis | Semi‐liquid | Digestion waste | Heat | 55 | 8.3 | 41.5 | Ugwuanyi et al. (1999) |
| Enterococcus faecalis | Semi‐liquid | Digestion waste | Heat | 60 | 6.61 | 33.05 | |
| Enterococcus faecalis | Semi‐liquid | Digestion waste | Heat | 55 | 4.72 | 23.6 | |
| Enterococcus faecalis | Semi‐liquid | Digestion waste | Heat | 60 | 5.24 | 26.2 |
3.6.2. Salmonella Senftenberg
Data on thermal inactivation of S. Senftenberg were extracted from the review of studies on the thermal resistance of salmonellae by Doyle and Mazzotta (2000). D‐values were extracted from studies carried out in different matrices and plotted against the tested temperature. Then, those DT‐values were used to estimate the times needed to inactivate 5 log10 units of S. Senftenberg as a function of the treatment temperature (Figure 5). The analysis included thermal inactivation in different products (i.e. liquid whole eggs, liquid egg yolks, liquid egg whites, raw milk, ground beef, chocolate and culture media).
Figure 5.
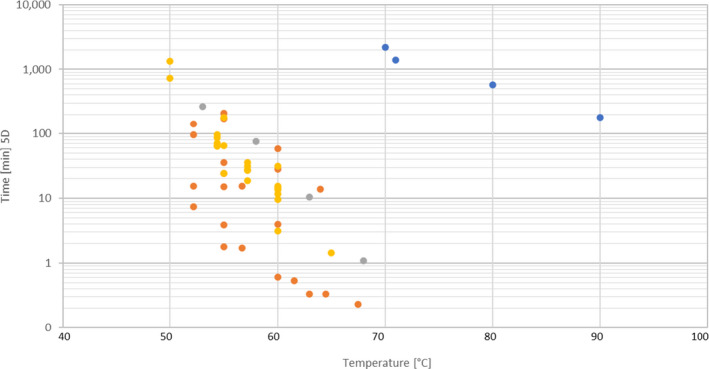
- Orange: liquid food product; grey: solid food product; yellow: liquid culture media; blue: semi‐liquid food product.
It is generally acknowledged that water activity (aw) influences thermal inactivation of microorganisms, and that in matrices with high water content, the resistance of microorganisms to thermal inactivation is lower (Syamaladevi et al., 2016). It is also well known that some components of the matrices such as fat can have a protective effect on bacteria subjected to thermal treatments. This effect was also evident in the thermal inactivation data set from Doyle and Mazzotta (2000), with S. Senftenberg showing D60‐values in high aw matrices ranging from 0.122 min in raw milk to 11.8 min in liquid egg yolks, while in chocolate it showed a D90‐value of 36 min (Doyle and Mazzotta, 2000). This provides evidence that the physico‐chemical characteristics of the ABP or the raw materials used as feedstock for the production of the derived products under assessment will impact on the levels of reduction achieved. Details of the data points used to produce Figure 5 are displayed in Table A.2 of Appendix A.
3.6.3. Viruses
Nims and Plavsic (2013d) conducted a systematic review analysis comparing four different viral families for their susceptibility to heat inactivation. Within the four viral families analysed, they found that Parvoviridae is by far the most heat‐resistant viral family followed by Caliciviridae and Picornaviridae. However, their review is not extensive and does not include all the viral families of the hazards identified in chapter 3.5.
The results of the ELS, conducted on the viral families selected in the hazard identification following the approach described in Section 3.5, produced the results presented in Table 5.
Table 5.
Results of the ELS and screening of the viral hazards and their thermal and/or chemical inactivation
| Viral hazard | Hits (reviews) | Y* | N* | D |
|---|---|---|---|---|
| Bovine papillomavirus and cottontail rabbit papillomavirus | 21 (1) | 0 | 20 | 1 |
| Swine vesicular disease virus | 65 (2) | 19 | 38 | 8 |
| Senecavirus A | 19 (1) | 2 | 12 | 5 |
| Ungulate tetraparvovirus | 38 (5) | 11 | 24 | 3 |
| Chicken anaemia virus | 27 (0) | 2 | 25 | 0 |
| Duck circovirus | 8 (1) | 7 | 0 | 1 |
| Bluetongue virus | 13 (0) | 0 | 12 | 1 |
Y: Yes (inactivation data available); N: No (no presence of in activation data) D: doubtful (not clear from the title and abstract if inactivation data will be available).
In the following sections, the data obtained by the literature review are presented in tabular form and/or through the graphical representation of the estimated times needed to achieve a 3 log10 reduction of infectious virus per viral family, calculated from the D‐values, in those cases where enough data points were retrieved.
3.6.3.1. Papillomaviridae
Data on the thermal inactivation of bovine papillomavirus and cottontail rabbit papillomavirus were not available in the reviewed papers. Only one study on human papillomavirus (HPV11) was retrieved and described the complete elimination of infectivity after a 60‐min treatment at 60°C (Smith et al., 1993). Summarised data from the references identified in the literature for Papillomaviridae are displayed in Table A.3 of Appendix A.
Table A.3.
Summarised data from the references identified in the literature review on thermal inactivation for Papillomaviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D(min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Human papillomavirus (HPV11) | Dermal tissue of neonatal foreskins | – | Heat | 60 | 60 | Total inactivation | – | Smith et al. (1993) |
| – | Heat | 37 | 60 | Infectivity retained | – |
3.6.3.2. Parvoviridae
Ungulate tetraparvovirus (Parvoviridae family) was identified as a viral hazard for wool and hair (group 5). However, data on the thermal inactivation of Ungulate tetraparvovirus were not available in the reviewed papers; therefore, data on other viruses of the family Parvoviridae were taken into consideration.
The data extracted included mainly thermal reduction data for canine, porcine or bovine parvovirus, derived from studies undertaken in a wide range of matrices, including human serum protein solution, human serum albumin, human plasma, manure, water and culture media. It is stated in the literature that Parvovirus B19 seems to be more susceptible to inactivation compared to other parvoviruses (Yunoki et al., 2003).
For Parvoviridae, the time–temperature combinations providing > 3 log10 reductions are more intense than those reported for other viral families (e.g. 112°C for 0.5 min for canine parvovirus in water; 101°C for 0.5 min in water for bovine parvovirus; 117°C for 0.5 min for minute virus of mice (MVM) in water; or 196°C for 0.5 min for mice virus in culture media) (Nims and Plavsic, 2013c).
The times required to achieve a 3 log10 reduction at different temperatures, extracted or calculated from selected references of the literature review for Parvoviridae are displayed in Figure 6. Summarised data from the references identified in the literature review for Parvoviridae are displayed in Table A.4 of Appendix A.
Figure 6.
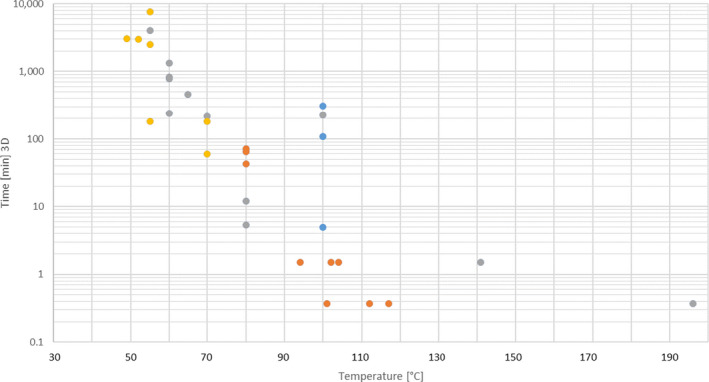
- Orange: water; grey: culture media; yellow: semi‐solid; blue: dried lyophilisate.
Table A.4.
Summarised data from the references identified in the literature review on thermal inactivation for Parvoviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D (min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Canine parvovirus (CPV) | Human serum protein solution | 105.5 TCID50 mL−1 | 103 | 1.5 | Total inactivation | Lelie et al. (1987) | ||
| 105 TCID50 mL−1 | 65 | 10 | 1.3 log TCID50 mL−1 | |||||
| 104.9 TCID50 mL−1 | 65 | 40 | 2.3 log TCID50 mL−1 | |||||
| 105.5 TCID50 mL−1 | 65 | 600 | Total inactivation | |||||
| Porcine parvovirus (PPV) | Manure (25% pig manure and 75% cow manure) and bleaching clay | 105.7 TCID50 50 mL−1 | 70 | 60 | 0.6 log10 | Lund et al. (1996) | ||
| 55 | 660 | 4 log10(Initial) | ||||||
| 55 | 3,240 | 4 log10 (Terminal) | ||||||
| Porcine parvovirus (PPV) | Manure, bleaching clay and household waste | 105.7 TCID50 50 mL−1 | 70 | 60 | 1.4 log10 | |||
| 55 | 720 | 4 log10 (Initial) | ||||||
| 55 | 3,240 | 4 log10 (Terminal) | ||||||
| Porcine parvovirus (PPV) | Manure and bleaching clay | 105.7 TCID50 50 mL−1 | 55 | 8,880 | 4 log10 | |||
| Bovine parvovirus (BPV) | Human plasma | Dry heat | 100 | 300 | 4 log10 | Bräuniger et al. (2000) | ||
| Moist heat | 60 | 600 | 4 log10 | |||||
| Parvovirus B19 | Human serum albumin | 60 | 10 | > 4 log10 | Blümel et al. (2002) | |||
| Porcine parvovirus (PPV) | Human serum albumin | 60 | 60 | Infectivity retained | Blümel et al. (2002) | |||
| Canine parvovirus (CPV) | Human serum albumin | 8.2 log10 TCID50 mL−1 | 60 | 60 | 0.7 log10 | 276.75 | Yunoki et al. (2003) | |
| 60 | 300 | 1.5 log10 | ||||||
| 60 | 600 | 2.5 log10 | ||||||
| 8.1 log10 TCID50 mL−1 | 60 | 60 | 0.3log10 | |||||
| 60 | 300 | 1.2log10 | ||||||
| 60 | 600 | 2.4 log10 | ||||||
| Canine parvovirus (CPV) | 0.5% Urinastatin solution | 8.9 log10 TCID50 mL−1 | 60 | 60 | 1.6 log10 | 80 | ||
| 60 | 300 | 5.1 log10 | ||||||
| 60 | 600 | > 6.4 log10 | ||||||
| Canine parvovirus (CPV) | 0.5% Urinastatin solution | 8.2 log10 TCID50 mL−1 | 60 | 60 | 1.3 log10 | |||
| 60 | 300 | 3.8 log10 | ||||||
| 60 | 600 | 5.7 log10 | ||||||
| Parvovirus B19 | Heated in liquid | 60 | 2–6 log10 | |||||
| Porcine parvovirus | Untreated mixed waste | Heat under laboratory conditions | 55 | 60 | 60 | Sahlström et al. (2008) | ||
| 70 | 30 | 19.8 | ||||||
| Parvovirus, avian strains | 65 | 30 | Infectivity retained | EFSA BIOHAZ Panel (2011) | ||||
| Bovine parvovirus (BPV) | Dry heat | 95 | 120 | Infectivity retained | EFSA BIOHAZ Panel (2011) | |||
| Canine parvovirus (CPV) | 80 | 420 | Infectivity retained | EFSA BIOHAZ Panel (2011) | ||||
| Minute virus of mice (MVM) | Culture media | 141 | 0.5 | 1 log10 | Nims and Plavsic (2013c) | |||
| 196 | 0.5 | 4 log10 | ||||||
| Minute virus of mice (MVM) | Water | 104 | 0.5 | 1 log10 | ||||
| 117 | 0.5 | 4 log10 | ||||||
| Bovine parvovirus (BPV) | Water | 94 | 0.5 | 1 log10 | ||||
| 101 | 0.5 | 4 log10 | ||||||
| Canine parvovirus (CPV) | Water | 102 | 0.5 | 1 log10 | ||||
| 112 | 0.5 | 4 log10 | ||||||
| Parvoviridae spp. | 110 | 0.5 | 1 log10 (b) | |||||
| Parvoviridae spp. | 131 | x0.5 | 4 log10 (b) | |||||
| Porcine parvovirus (PPV) | Saline solution | 70 | 72 | 1 log10 | Elving et al. (2014) | |||
| 6.7 log10 TCID50 g−1 | 70 | 60 | 0.9 log10 | |||||
| 55 | 1 log10 | 1,372 | ||||||
| Porcine parvovirus (PPV) | Dairy cow faeces | 49 | 1 log10 | 1,019 | ||||
| 52 | 1 log10 | 1,006 (CI95% 828.6–1,280.4) | ||||||
| 52 | 3,840 | 3 log10 | ||||||
| 55 | 1 log10 | 650 (CI95% 531–839.4) | ||||||
| 55 | 2,520 | 3 log10 | ||||||
| Minute virus of mice (Protoparvovirus) | Culture medium | (35, 45, 60, 100°C) | 4 (D80)(a) | Nims and Zhou (2016) | ||||
| Minute virus of mice (Protoparvovirus) | Water | (70, 80, 90°C) | 14.3 (D80)(a) | |||||
| Canine parvovirus (Protoparvovirus) | Water | (56, 80, 100°C) | 21.4 (D80)(a) | |||||
| Bovine parvovirus (Bocaparvovirus) | Water | (75, 80, 85, 90°C) | 23.6 (D80)(a) | |||||
| Parvovirus B19 (Erythroparvovirus) | 5% Albumin | (52, 53, 54, 55.5, 57.5, 59, 60°C) | < 0.017 (D80)(a) | |||||
| Parvovirus B19 (Erythroparvovirus) | Culture medium | (50, 60, 70°C) | 1.8 (D80)(a) |
D‐values from Nims and Zhou (2016) were estimated based on the reported data for other temperatures.
Average temperature value for 1 log10 and 4 log10 reduction in 30 s.
3.6.3.3. Picornaviridae
Table A.5 of Appendix A shows the results of the literature search on swine vesicular disease virus and Senecavirus A, according to the search strategy presented in Section 3.5. However, specific data on thermal inactivation were only available in the literature for swine vesicular disease, not for Senecavirus A. The data retrieved included mainly thermal reduction data for enterovirus, swine vesicular disease virus, poliovirus, infectious avian encephalomyelitis virus and FMDV, derived from studies undertaken in a wide range of matrices, including wastewater sludge, saline solution, manure, slurry, faeces, milk and culture media. Some of the studies in the literature reported > 3 log10 reductions at time/temperature combinations of relevance for the mandate, like 60 min at 70°C in saline solution or manure for bovine enterovirus (Lund et al., 1996). In the particular case of swine vesicular disease virus, complete inactivation has been reported after treatments of less than 5 min at temperatures ranging from 56°C to 60°C in pig slurry (Turner et al., 1998; Turner and Williams, 1999).
Table A.5.
Summarised data from the references identified in the literature review on thermal inactivation for Picornaviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D(min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Feline picornavirus | Feline kidney tissue | 105–107 TCID50 0.1 mL−1 | 50 | 30 | Inactivation | Flagstad (1972) | ||
| Picornavirus/poliovirus | Dewatered wastewater sludge | 3*109–8*109 PFU mL−1 | Heat due to composting | 47 | 40–50 | 3 log10 | Ward and Ashley (1978) | |
| s.c. 5% w/w | 51 | ˜ 20 | 3 log10 | |||||
| s.c: 80% w/w | 47 | 40–50 | 1 log10 | |||||
| 51 | ˜ 20 | 1 log10 | ||||||
| Bovine enterovirus | Saline solution | 107.8–108.2 TCID50 200 mL−1 | 70 | 60 | > 3.5 log10 | Lund et al. (1996) | ||
| 55 | 138 | 4 log10 | ||||||
| Bovine enterovirus | Manure with bleaching clay | 107.8–108.2 TCID50 200 mL−1 | *pH 8 | 70 | 60 | 3.8 log10 | ||
| 35 | 96 | 4 log10 | ||||||
| 55 | < 0.5 log10 | |||||||
| Bovine enterovirus | Manure, bleaching clay and household waste (20%) | 107.8–108.2 TCID50 200 mL−1 | *pH 8 | 70 | 60 | 3.6 log10 | ||
| 55 | < 0.5 log10 | |||||||
| Swine vesicular disease virus | Pig slurry | 107 PFU mL−1 | Heat | 60 | 1.5 | Total inactivation | Turner et al. (1998) | |
| Swine vesicular disease virus | Pig slurry s.c (TS) 1.2–20% | 4.7–5.5 log10 PFU mL−1 | *pH 7.5–8 | 50–55 | Total inactivation | Turner et al. (1999) | ||
| *pH 6.4 | 55–60 | Total inactivation | ||||||
| Swine vesicular disease virus | Pig slurry s.c. 2–5% | 107.7 PFU mL−1 | Heat | 50–60 | Total inactivation | Turner and Williams (1999) | ||
| 56 | 5 | 6 log10 | ||||||
| 60 | 2 | Total inactivation | ||||||
| Poliovirus 1 (strain Lsc‐2ab) and five environmental isolates (coxsackievirus B4, coxsackievirus B5, echovirus 6 and two enteroviruses) | Sludge and raw sewage | 60 | 30 | 4.3 log10–5.4 log10 | Mocé‐Llivina et al. (2003) | |||
| Poliovirus 1 (Enterovirus) | Cell cultures | 103 PFU mL−1 | Heat | 72 | 0.09 | 1 log10 | Nuanualsuwan and Cliver (2003) | |
| Enterovirus spp. | ‘Faeces and food waste mix’ with and without urine | Composting process | 40–65 | ≥ 11 days | ≥ 12 log10 | Vinnerås et al. (2003) | ||
| Enterovirus spp. | Wastewater sludge | 3.4–167 MPNCU g−1 (dry matter) | Mesophile anaerobic digestion and heat‐pressure (19–21 bar). Liming: quicklime 50% dry matter; mixture homogenised after cooling.* pH 12.5–13 | 195 | 100 | Total inactivation | Monpoeho et al. (2004) | |
| Infectious avian encephalomyelitis virus (AEV) and avian enterovirus‐like viruses | Heat | 56 | 60 | Infectivity retained | EFSA BIOHAZ Panel (2011) | |||
| Foot and Mouth Disease Virus | Culture medium | pH 7.5 | 49 | 60 | Bachrach et al.(1957) | |||
| 55 | 2 | |||||||
| 61 | 0.5 | |||||||
| Foot and Mouth Disease Virus | Culture medium | 55 | 0.71 | Bachrach (1959) | ||||
| Foot and Mouth Disease Virus | Slurry | pH 7.5 | 55 | 3.23 | Turner et al. (2000) | |||
| 60 | 2.33 | |||||||
| 65 | 0.94 | |||||||
| 67 | 0.45 | |||||||
| Foot and Mouth Disease Virus | Culture medium | 55 | 2 | |||||
| 60 | 2.7 | |||||||
| 65 | 1.14 | |||||||
| 67 | 0.53 | |||||||
| 70 | 0.29 | |||||||
| Foot and Mouth Disease Virus | NS | pH 7.5 | 61 | 0.5 | Pharo (2002) | |||
| 55 | 2 | |||||||
| 49 | 60 | |||||||
| Foot and Mouth Disease Virus | Milk | 63 | 15 | Aly and Gaber (2007) | ||||
| 72 | 0.13 | |||||||
| Foot and Mouth Disease Virus | PBS buffer | 50 | 12.2 | Kamolsiripichaiporn et al. (2007) | ||||
| 50 | 21.25 | |||||||
| 60 | 0.27 | |||||||
| 60 | 0.7 | |||||||
| 70 | 0.1 | |||||||
| 70 | 0.18 | |||||||
| 80 | 0.05 | |||||||
| 80 | 0.1 | |||||||
| 90 | 0.03 | |||||||
| 90 | 0.05 | |||||||
| 100 | 0.03 | |||||||
| 100 | 0.05 | |||||||
| Foot and Mouth Disease Virus | Milk | pH 6.7 | 72 | 0.06 | Ryan et al. (2008) | |||
| pH 7.6 | 72 | 0.18 | ||||||
| 85 | 0.09 | |||||||
| Foot and Mouth Disease Virus | Aqueous media | 70 | 0.2 | Donaldson et al. (2011) | ||||
| 56 | 2 | |||||||
| Foot and Mouth Disease Virus | Meat slurry | pH 6.6 | 40 | 0.97 | Gubbins et al. (2016) | |||
| 50 | 0.59 | |||||||
| 60 | 0.49 | |||||||
| 70 | 0.7 | |||||||
| 68 | 0.57 | |||||||
| Foot and Mouth Disease Virus | Mix (meat slurry and dry meal, adjusted pH) | RH 28%, pH 6.7 | 40 | 2.82 | ||||
| 50 | 1.24 | |||||||
| 60 | 1.23 | |||||||
| 70 | 1.52 | |||||||
| 79 | 0.06 | |||||||
| 79 | 0.02 | |||||||
| Foot and Mouth Disease Virus | Bovine tongue epithelium | pH 7.6 | 79 | 0.02 | ||||
| Foot and Mouth Disease Virus | NS | 49 | 60 | Williams (2017) | ||||
| 61 | 0.05 |
MPNCU (most probable number of cytopathic unit).
Combination of thermal and chemical treatment. D‐values on Foot and Mouth Disease Virus were extracted from the REFRESH study (Hayrapetyan et al., 2019).
S.c.: solid content.
NS: not specified.
The times required to achieve a 3 log10 reduction at different temperatures, extracted or calculated from selected references of the literature review for Picornaviridae are displayed in Figure 7. Summarised data from the references identified in the literature review for Picornaviridae are diplayed in Table A.5 of Appendix A.
Figure 7.
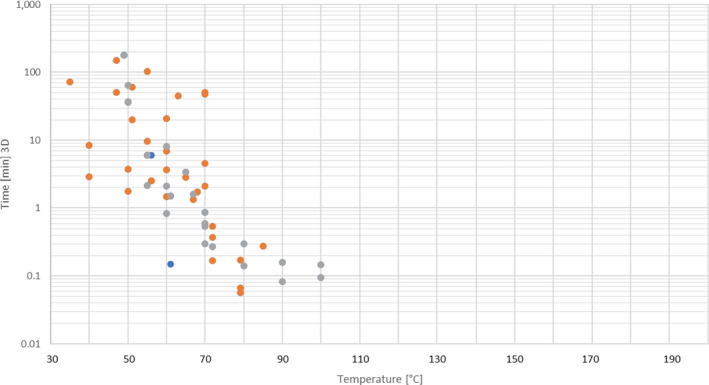
- Orange: liquid; grey: culture media; blue: not specified.
3.6.3.4. Anelloviridae
The data extracted from the literature on thermal inactivation of viruses belonging to the Anelloviridae family exclusively included thermal reduction data for chicken anaemia virus (CAV) obtained in human albumin, minced meat or chicken by‐products. According to the literature, > 3 log10 reductions are reached at time–temperature combinations of relevance for the mandate, like 30 min at 75°C in human albumin (Welch et al., 2006).
The times to achieve a 3 log10 reduction at different temperatures, extracted or calculated from the references of the literature review for Anelloviridae (CAV), are displayed in Figure 8. Summarised data from the references identified in the literature review for Anelloviridae (Chicken anaemia virus) are displayed in Table A.6 of Appendix A.
Figure 8.
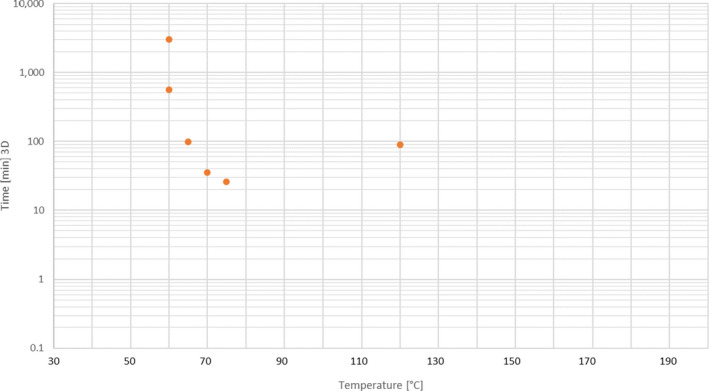
- Orange: liquid (human albumin).
Table A.6.
Summarised data from the references identified in the literature review on thermal inactivation for Anelloviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D(min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Chicken anaemia virus (strain Cux‐1AV) | Minced meat from CAV‐experimentally infected chicken carcassa | 5.4 log10 TCID50 g−1 | Heating in a stirred water bath | 70 | 4 | Infectivity retained | Urlings et al. (1993) | |
| 80 | 4 | Infectivity retained | ||||||
| 90 | 4 | Infectivity retained | ||||||
| 95 | 4 | Infectivity retained | ||||||
| 95 | 10 | Infectivity retained/Inactivation | ||||||
| 95 | 30 | Total inactivation | ||||||
| 100 | 10 | Total inactivation | ||||||
| 100 | 30 | Total inactivation | ||||||
| Chicken anaemia virus (strain Cux‐1AV) | Minced meat from CAV‐experimentally infected chicken carcassa + 4% w/w dextrose | Heating in a stirred water bath | 90 | 4 | Infectivity retained | |||
| 95 | 10 | Infectivity retained/Inactivation | ||||||
| 95 | 30 | Total inactivation | ||||||
| 100 | 10 | Total inactivation | ||||||
| 100 | 30 | Total inactivation | ||||||
| Heating in a stirred water bath +106 cfu Lactobacillus plantarum under vacuumb | 90 | 4 | Infectivity retained | |||||
| 95 | 10 | Infectivity retained | ||||||
| 95 | 30 | Total inactivation | ||||||
| 100 | 10 | Total inactivation | ||||||
| 100 | 30 | Total inactivation | ||||||
| Chicken anaemia virus (CAV) | Human albumin | Virus:product ratio 1:10.4 | Pasteurisation in a water bath | 60 | 1,440 | 1.42 log10 | Welch et al. (2006) | |
| 60 | 30 | 0.16 log10 | ||||||
| 65 | 30 | 0.91 log10 | ||||||
| 70 | 30 | 2.58 log10 | ||||||
| 75 | 30 | 3.5 log10 | ||||||
| Dry heat treatment | 80 | 4,320 | 1.25 log10 | Welch et al. (2006) | ||||
| 120 | 30 | 1 log10 | ||||||
| Chicken anaemia virus (CAV) | 80 | 30 | Infectivity retained | EFSA BIOHAZ Panel (2011) | ||||
| 100 | 15 | Total inactivation |
After removal of feathers, skin and feet.
At 20°C for 7 days (fermentation).
3.6.3.5. Circoviridae
Although the viral hazard identified for feathers and down (Group 6) is Duck circovirus (family Circoviridae), the data extracted exclusively included thermal reduction data for porcine circovirus in human albumin. According to the literature, certain time/temperature combinations of relevance for the mandate (e.g. 30 min at 75°C, or 30 min at 120°C with dry heat) produce log10 reductions < 3 log10 for this virus (Welch et al., 2006). These authors investigated the resistance of Porcine circovirus 2 (PCV2) and Chicken anaemia virus (CAV). The dry‐heat treatment at 120°C for 30 min led to approximately 1‐log10 reduction in infectivity.
The time to achieve a 3 log10 reduction at different temperatures, extracted or calculated from the references of the literature review for Circoviridae, are displayed in Figure 9. Summarised data from the references identified in the literature review for Circoviridae are displayed in Table A.7 of Appendix A.
Figure 9.
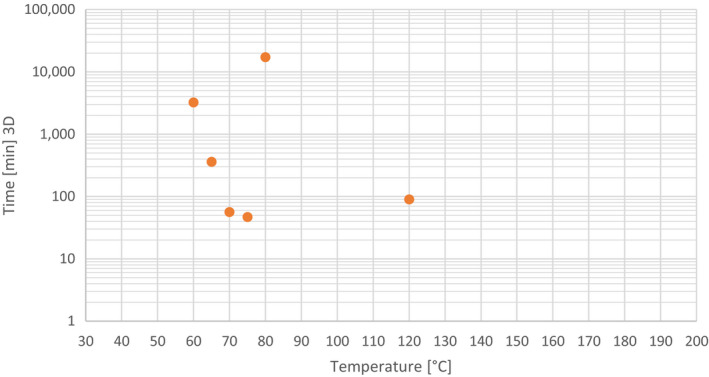
- Orange: liquid (human albumin).
Table A.7.
Summarised data from the references identified in the literature review on thermal inactivation for Circoviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D(min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Porcine circovirus 2 (PCV2) | Human albumin | Virus:product ratio 1:10.4 | Pasteurisation in a water bath | 60 | 1440 | 1.33 log10 | Welch et al. (2006) | |
| 65 | 30 | 0.25 log10 | ||||||
| 70 | 30 | 1.59 log10 | ||||||
| 75 | 30 | 1.92 log10 | ||||||
| Dry heat | 80 | 4,320 | 0.75 log10 | |||||
| 120 | 30 | 1 log10 | ||||||
| Porcine circovirus 2 (PCV2) | Dry heat | 120 | 30 | Infectivity retained | EFSA BIOHAZ Panel (2011) | |||
| Moist heat | 75 | 15 | Infectivity retained |
3.6.3.6. Reoviridae
Data on the Bluetongue virus (BTV) were not available from the identified literature. The data extracted were exclusively thermal reduction data for avian rotaviruses and reoviruses at mild heating temperatures of 56–60°C, with variable results. Summarised data from the references identified in the literature review for Reoviridae are provided in Table A.8 of Appendix A.
Table A.8.
Summarised data from the references identified in the literature review on thermal inactivation for Reoviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | Level of inactivation | D(min) | Reference |
|---|---|---|---|---|---|---|---|---|
| Avian rotaviruses | Heat | 56 | 30 | 2 log10 | EFSA BIOHAZ Panel (2011) | |||
| Avian reoviruses | Heat | 60 | 480–600 | Infectivity retained |
3.7. Chemical and thermo‐chemical inactivation
The application of the search string as described in Table A.1 of Annex A produced 74 hits, including two review articles, related to chemical and thermo‐chemical inactivation of biological hazards and indicator microorganisms. The screen of title and abstracts looking for data on chemical inactivation of the indicator microorganisms resulted in 38 references with potential data to extract. Out of these, 36 references were in English and reviewed in full. Data from 21 papers were extracted and a summary is provided in Tables 6, 7, 8, 9, 10, 11. In the other 15 papers, either data on parameters of the treatment applied or quantification of the reduction of the indicator microorganisms were not available. In various of these studies, the chemical process was accompanied by a thermal treatment at temperatures ranging from 50°C to 70°C. Therefore, in these instances, the inactivation level attained is due to a combined thermo‐chemical effect. It is noteworthy that some of the industrial processes under assessment based on chemical inactivation (e.g. liming) are exothermic processes which release heat, causing a progressive increase in temperature in an uncontrolled manner. For example, as stated in EFSA BIOHAZ Panel (2010), the reaction between quicklime and the water of the manure is exothermic (1,140 kJ/kg of CaO) and at the correct dosage rate is sufficient to raise the temperature of the manure undergoing treatment of 70°C for 30 min or 60°C for 60 min. Similarly, Paluszak et al. (2006) reported an increase of the temperature up to 60°C only when the concentration of quicklime was at least 20% in sludge, causing a reduction of > 5 log10 of S. Senftenberg in 30 min. If the concentration of quicklime was 10% or 5%, the temperature never exceeded 30°C, not adding any thermal effect to the inactivation. The assessment of chemical treatments that do not specify the minimum residence time but only the minimum pH to be reached is more uncertain about their efficacy to reduce/inactivate microbiological indicators.
Table 6.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for E. faecalis
| Matrix/substrate | Indicator pathogen | Initial load | Treatment | Level of inactivation | Reference |
|---|---|---|---|---|---|
| Surface dentine | E. faecalis (biofilm) | 4.49 (STD 1.66) log CFU/dentin chips | CH: calcium hydroxide + NaOCl: sodium hypochlorite = pH 12.6 (STD: 0.03). 37°C 7 days | 2 log10 | Shokraneh et al. (2014) |
| Surface dentine | E. faecalis (biofilm) | 4.49 (STD 1.66) log CFU/dentin chips | CH: calcium hydroxide + distilled water = pH 12.63 (STD: 0.02) 37°C 7 days | 1.48 log10 | Shokraneh et al. (2014) |
| Horse manure/soil mixtures | E. faecalis | 107 CFU·g−1 manure | 2% of Ca(OH)2 at 14°C for 2 days, pH ˜ 12 | Total inactivation (up to 5 log10 reduction; Limit of detection: 2 log10) in clay soils (not in sandy soils: still detectable after 15 days) | Nyberg et al. (2011) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | E. faecalis | n/a | Maximum particle size: 12 mm. quicklime (CaO) 30min 70°C at pH 12 | > 5 log10 | EFSA BIOHAZ Panel (2010) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | E. faecalis | n/a | Maximum particle size: 12 mm. quicklime (CaO) 60 min 60°C at pH 12 | > 5 log10 | EFSA BIOHAZ Panel (2010) |
Table 7.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for S. Senftenberg
| Matrix/substrate | Indicator pathogen | Initial load | Treatment | Level of inactivation or kinetic parameter | Reference |
|---|---|---|---|---|---|
| Orange juice | S. enterica serovar Senftenberg CECT 4384 (Salmonella Senftenberg) | About 108 CFU·mL−1 | pH conditions (pH 2.5, adjusted with acid) at room temperature | D = 12.2–19.1 min | Álvarez‐Ordóñez et al. (2009) |
| Apple juice | S. enterica serovar Senftenberg CECT 4384 (Salmonella Senftenberg) | About 108 CFU·mL−1 | pH conditions (pH 2.5, adjusted with acid) at room temperature | D = 20.6–54.9 min | Álvarez‐Ordóñez et al. (2009) |
| Orange juice | S. enterica serovar Senftenberg CECT 4384 (Salmonella Senftenberg) | About 108 CFU·mL−1 | Multiple measurements for thermal inactivation at 55, 58 and 63°C in orange juice (pH 2.5; adjusted with acid) from bacteria grown in buffered or nonacidified BHI at room temperature | D55 = 0.37–1.05 min, D58 = 0.11–0.34 min, D63 = 0.025–0.072 min | Álvarez‐Ordóñez et al. (2009) |
| Apple juice | S. enterica serovar Senftenberg CECT 4384 (Salmonella Senftenberg) | About 108 CFU·mL−1 | Multiple measurements for thermal inactivation at 55, 58 and 63°C in apple juice (pH 2.5; adjusted with acid) from bacteria grown in buffered or non‐acidified brain heart infusion (BHI) at room temperature | D55 = 0.43–1.05 min, D58 = 0.19–0.41 min, D63 = 0.034–0.086 min | Álvarez‐Ordóñez et al. (2009) |
| Sludge | S. Senftenberg 775W | 106–107 CFU·g−1 | 5, 10 and 20% CaO up to 24 h, 5%: pH 12.5–13.2 10%: pH 13–13.2 20%: pH 13–13.6 | > 5log10 30 min in 20% CaO 5 log10 30 min 10% CaO 5 log10 1h with 5% CaO | Paluszak et al. (2006) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | S. Senftenberg | n/a | Maximum particle size: 12 mm. quicklime (CaO) 30min 70°C at pH 12 | > 5 log10 | EFSA BIOHAZ Panel (2010) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | S. Senftenberg | n/a | Maximum particle size: 12 mm. quicklime (CaO) 60min 60°C at pH 12 | > 5 log10 | EFSA BIOHAZ Panel (2010) |
Table 8.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for Ascaris sp
| Matrix/substrate | Indicator pathogen | Initial load | Treatment | Level of inactivation | Reference |
|---|---|---|---|---|---|
| Sludge | Non‐larval Ascaris (Ascaris lumbricoides, Ascaris suum) | 215 eggs (35 per genera → 70 Ascaris eggs) per 2 g Total Solids (TS) | A 15% lime (CaO) (pH 12.5), humidity 90% for 10 months | Total inactivation | Maya et al. (2010) |
| Sludge | Non‐larval Ascaris (Ascaris lumbricoides, Ascaris suum) | 215 eggs (35 per genera → 70 Ascaris eggs) per 2 g TS | A 15% lime (CaO) (pH 12.5), humidity 80% for 9 months | Total inactivation | Maya et al. (2010) |
| Sludge | Non‐larval Ascaris (Ascaris lumbricoides, Ascaris suum) | 215 eggs (35 per genera → 70 Ascaris eggs) per 2 g TS | A 20% lime (CaO) (pH 12.5) humidity 90% for 8 months | Total inactivation | Maya et al. (2010) |
| Sludge | Non‐larval Ascaris (Ascaris lumbricoides, Ascaris suum) | 215 eggs (35 per genera → 70 Ascaris eggs) per 2 g TS | A 20% lime (CaO) (pH 12.5) humidity 80% for 7–8 months | Total inactivation | Maya et al. (2010) |
| Human excreta | Ascaris suum eggs | 24 bags, each bag contained approximately 20,000 A. suum eggs in each heap of material | Lime pH values ranging between 9.4 and 11.6 | < 1% after 105–117 days of storage | Jensen et al. (2009) |
| Class B biosolids (sludge) | Ascaris lumbricoides | 3,000 A. lumbricoides ova | Calcium hydroxide pH 12.0 for 2 h, then 0.1 N HCl was added drop by drop until a pH value of 11.5 was achieved and maintained for the duration of the experiment (72 h). 28°C | There was no significant difference between viability of control and test samples at all time points. Ascaris ova remained viable after 72 h liming | Bean et al. (2007) |
| Artificially contaminated milk of lime | Ascaris eggs | 500 Ascaris eggs | pH 12.6, Temp 50°C, 55°C and 60°C, samples in intervals | Inactivation after 70 min at 50°C, 5 min at 55°C and 2 min at 60°C | Capizzi‐Banas et al. (2004) |
| Sewage sludge | Ascaris eggs | Four silk bags containing 106 Ascaris eggs in each | Lab‐scale (pH > 12) and full‐scale (pH not measured), 22% to 26% CaO/TS. Other combinations of quicklime, slaked lime, etc., in lab experiments | Total inactivation after 75 min at 55°C and 5 min at 58°C | Capizzi‐Banas et al. (2004) |
| Dewatered raw sludge | Ascaris suum eggs | Approx. 2,400 eggs/g from naturally infected pigs | Temperature > 80°C for ≥ 50 min, a pH 12.4 due to lime conditioning and also probably high ammonia content (not measured during test) | No viable eggs after: 45 min at 61–62.5°C (thermophilic aerobic pretreatment) 15 min at 65–66.5°C (pre‐pasteurisation) 50 min at 80°C (lime conditioning and thermal vacuum drying in membrane filter press) | Paulsrud et al. (2004) |
| Secondary raw sludge and thickened raw sludge | Ascaris eggs | Approx. 16,000 eggs/L in secondary and 300–400 eggs/g in dehydrated (thickened) sludge | Criteria: pH ≥ 12, minimum 2 h Lime dosages tested were in the range between 0.6 and 32 g/L for raw sludge with a total solids concentration of 1.0–4.5% (7 g/L lime was sufficient to fulfil this criteria), dehydrated using a turbine centrifuge | 4–18 eggs/g in lime stabilised and dehydrated sludge | Mijaylova Nacheva et al. (2002) |
| Wastewater sludge | Helminths eggs (90% of them Ascaris spp.) | approx. 60 eggs/g TS | Dewatered sludge, 40% (w/w) quicklime for 2 h, pH > 12, max. temp registered 37.8°C. | Reduction of helminths from 60/gof TS to 6–> 90% reduction | Jimenez et al. (2000) |
| Dewatered sewage sludge (mix of primary and secondary sludge, 20% dry matter) | Ascaris suum eggs | 8000 eggs/mL in free egg batch, 2,000,000 eggs/nylon bag in bag batch | 10% w/w quicklime as 85% CaO increased the temp to 45°C, pH > 12, left at room temperature in the dark | No embryonation anymore of free eggs after 10 weeks and of eggs in nylon bags after 12 weeks | Eriksen et al. (1996) |
| Sewage sludge | Ascaris eggs | n/a | Different pasteurisation treatments, one experienced with liming | Ascaris eggs destroyed at 60–70°C 30min 50°C 54 h, 70°C 2 h in unslaked lime | Strauch (1983) |
| Untreated/raw faeces | Ascaris lumbricoides eggs | 119 (total) and 94 (viable) eggs/g total solid | Untreated/raw faeces + lime (1:3), pH (mean during 40 days) 10.2 | Total inactivation of viable eggs after 30 days | Endale et al. (2012) |
| Dewatered biosolids (sludge) | Ascaris suum eggs | Spiked with 106 eggs to achieve 2000 eggs per effluent sample of 150 g (wet weight) | Mixed with calcium oxide (ratio lime: sludge 1:1 on dry weight basis), pH > 12, different Temp/Time/Pressure combinations | Total inactivation after 55°C 85 min 1 atm, 55°C 25 min 2 atm, 55°C 11 min 2.6 atm | Fitzmorris et al. (2007) |
| Sludge | Ascaris suum eggs | Bag containing 1 mL of egg suspension | 5, 10 and 20% CaO up to 24 h, pH ≥ 13 | All eggs inactivated after 6 h with 20% CaO | Paluszak et al. (2006) |
| Digested and dewatered biosolids (28–30% TS) | Ascaris suum eggs | About 200 eggs/treatment | 100 g lime/kg biosolid and 200g lime/kg biosolid, T:17.5°C first 69 days and 2.9°C afterwards, pH decreased over time (not shown) | 2 log10 reduction after 40 days, all eggs inactivated after 69 days | Abu‐Orf et al. (2004) |
| Dewatered biosolids: Waste Activated Sludge (Raw) | Ascaris | 519 larvae Ascaris. | Quicklime (CaO) pH > 12 (minimum 12.38) Temperature: 55°C Total solids 17.9% 40 min | 2.7 log10 reduction of viable Ascaris | Brisolara and Reimers (2013) |
| Aerobic sludge | Ascaris | < 1 ova/4 grams dry weight | Quicklime (CaO) pH > 12 (minimum 12.38) Temperature: 55°C Total solids 17.9% 40 min | 3–3.4 log10 reduction of viable Ascaris | Brisolara and Reimers (2013) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | Ascaris eggs | n/a | Maximum particle size: 12 mm. quicklime (CaO) 30 min 70°C at pH = 12 | > 3 log10 | EFSA BIOHAZ Panel (2010) |
| Dewatered pig (28.5% dry solid contents) and poultry (40% dry solid contents) manure | Ascaris eggs | n/a | Maximum particle size: 12 mm. quicklime (CaO) 60 min 60°C at pH 12 | > 3 log10 | EFSA BIOHAZ Panel (2010) |
Table 9.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for Parvoviridae
| Virus | Matrix/ | Initial load | Treatment | T(°C) | t(min) | pH | Level of inactivation | Reference |
|---|---|---|---|---|---|---|---|---|
| Porcine parvovirus (Protoparvovirus) | Buffer solution + pepsin (porcine gastric mucosa) | – | 8 | 1.7 | 3.3–4.4 log10 | Yang et al. (2020) | ||
| 1,440 | 1.7 | 4.5–5 log10 | ||||||
| Porcine parvovirus (Protoparvovirus) | Serum | Buffer solution | 480 | 1.7 | 3.33–4.35 log10 | |||
| 1,440 | 1.7 | 4.47–5.03 log10 | ||||||
| Porcine parvovirus (Protoparvovirus) | Porcine small intestine | 4 × 0.2 mL containing 107 PFU mL−1 | 0.18% peracetic acid 4.8% aqueous ethanol mixture (PES)a | RT | 8.3 | 1 log10 | Hodde and Hiles (2002) | |
| Parvovirus | Digested sludge | 10 kg/m3 (CaO) | 720 | Total inactivation | Strauch (1983) | |||
| Parvovirus | Dewatered pig (28.5% dry s.c) and poultry (40% dry s.c.) manure | n/a | Maximum particle size: 12 mm. quicklime (CaO) | 70 | 30 | 12 | > 3 log10 | EFSA BIOHAZ Panel (2010) |
| 60 | 60 | 12 | > 3 log10 | EFSA BIOHAZ Panel (2010) |
s.c.: solid content.
Table 10.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for Picornaviridae
| Virus | Matrix/substrate | Initial load | Treatment | T° (C) | t (min) | pH | Level of inactivation | Reference |
|---|---|---|---|---|---|---|---|---|
| Coxsackie A9 (Enterovirus) | Cell cultures | 20 | 15 | 3, 4, 5, 6, 7 | < 1 log10 | Nims and Zhou (2016) | ||
| Bovine enterovirus (Enterovirus E) | Cell cultures | 20 | 15 | 3, 4, 5, 6, 7 | < 1 log10 | |||
| Porcine enterovirus (Porcine teschovirus) | Cell cultures | 20 | 15 | 3, 4, 5, 6, 7 | < 1 log10 | |||
| Encephalomyocarditis (Cardiovirus) | Cell cultures | 20 | 15 | 3 | 1 log10 | |||
| 20 | 15 | 4, 5, 6, 7 | < 1 log10 | |||||
| Human rhinovirus 2 (Enterovirus) | Cell cultures | 20 | 15 | 3, 4, 5 | > 3 log10 | |||
| 20 | 15 | 6 | 1.3 log10 | |||||
| 20 | 15 | 7 | < 1 log10 | |||||
| Equine rhinovirus (Equine rhinitis A virus) | Cell cultures | 20 | 15 | 3, 4, 5 | > 4 log10 | |||
| 20 | 15 | 6, 7 | < 1 log10 | |||||
| Foot and mouth disease virus (Aphthovirus) | Cell cultures | 20 | 15 | 3, 4, 5, 6 | > 3 log10 (strain O‐1); > 4 log10 (strain A‐61) | |||
| 20 | 15 | 7 | 1.2 log10 (strain O‐1); 1.5 log10 (strain A‐61) | |||||
| Enteroviruses (polio 1, 2 and 3, coxsackie B2, B3, B4 and B5, and echo 9) | Mixed raw and humus sludge, surplus activated sludge, and sludge dewatered by centrifugation | Variable ˜ 149 ± 43 PFU g−1 | Sludge cake produced by filter pressing after lime‐copperas. 39% solid content. | 10–11 | Full inactivation of poliovirus | Goddard et al. (1982) | ||
| Picornaviruses (aichivirus (AiV), coxsackievirus A9 (CAV9), coxsackievirus B5 (CBV5), and human parechovirus (HPeV) | Foetal Rhesus monkey kidney cells, buffalo green monkey kidney, Vero cells | 1 week in 0.1 mol/L solutions of HEPESa at pH 4 and 7 prior to 21°C pressure treatment. | 4 4 | 1 log10 for AiV for pH 4 compared to pH 7. 3 log10 for HPeV 4 log10 for CAV9 at pH4 compared to pH7 | Kingsley et al. (2014) | |||
| Human rotaviruses and enteroviruses | Sewage | Up to 10,000 I.U./L | Removal from sewage – to sludge. Coagulation by Lime treatment | Full inactivation | Bosch et al. (1986) | |||
| Poliovirus | Raw sludge | 3 kg/m3 quicklime (CaO) | 30 | 12 | Total inactivation | Strauch (1983) | ||
| Poliovirus | Digested sludge | 10 kg/m3 quicklime (CaO) | 360 | Total inactivation | ||||
| Bovine enterovirus | Liquid sludge | Aerobic‐thermophilic stabilisation | 48 | 1,800 | 6.6 | Total inactivation | Strauch (1983) | |
| 1,320 | 9.2 | Total inactivation | ||||||
| Feline picornavirus | Feline kidney tissue | 105–107 TCID50/0.1 mL | RT | 180 | 2, 3, 4, 9 | < 0.5 TCID50/0.1 mL | Flagstad (1972) | |
| 5, 7 | 4.5 TCID50/0.1 mL | |||||||
| Swine vesicular disease virus | Pig slurry solid contents (2–5%) | 107.7 PFU mL−1 | 1.5% (w/v) NaOH or Ca(OH)2 | 4 | 2.5 | Total inactivation | Turner and Williams (1999) | |
| 22 | 2.5 | Total inactivation | ||||||
| Swine vesicular disease virus | Pig slurry | 107 PFU mL−1 | 2% NaOH and Ca(OH)2 | 4 | 2.5 or 5 | Total inactivation | Turner et al. (1998) | |
| 1.5% NaOH and Ca(OH)2 | 4 or 22°C | 2.5 | Total inactivation |
N‐2‐hydroxyethylpiperazine‐ N’‐2‐ethane sulfonic acid; Invitrogen.
Table 11.
Summarised data on chemical and thermo‐chemical inactivation from the references identified in the literature review for Reoviridae
| Virus | Matrix/substrate | Initial load | Treatment | T (°C) | t(min) | pH | Level of inactivation | Reference |
|---|---|---|---|---|---|---|---|---|
| Porcine reovirus | Porcine small intestine | 4 × 0.2 mL containing 107 PFU mL−1 | 0.18% peracetic acid/4.8% aqueous ethanol mixture (PES) | RT | 5.5 | 1 log10 | Hodde and Hiles (2002) | |
| Human rotaviruses | Sewage | Up to 10,000 I.U./L | Removal from sewage and transfer to sludge. Coagulation by Lime treatment | Total inactivation | Bosch et al. (1986) | |||
| Reovirus | Raw sludge | 3 kg/m3 quicklime (CaO) | 180 | Total inactivation | Strauch (1983) | |||
| Reovirus | Digested sludge | 10 kg/m3 quicklime (CaO) | 180 | Total inactivation |
RT: room temperature.
3.7.1. Enterococcus faecalis
In EFSA BIOHAZ Panel (2010), the lime stabilisation of dewatered pig and poultry manures (pH 12, 30 min at 70°C or 60 min at 60°C) led to a 5 log10 reduction of Enterococcus faecalis (EFSA BIOHAZ Panel, 2010). However, the level of inactivation when incorporating manure into a soil was dependent on soil texture, with total inactivation in clay soil but not in sandy soil (still detectable after 15 days) (Nyberg et al., 2011). The high pH was critical for the inactivation of Enterococcus faecalis in the mixture of manure and soil and the minimum requirement for soil pH was 11. In a very different setting, E. faecalis is a common bacterium in persistent infections of dental surfaces. Treatments with calcium hydroxide or calcium hydroxide + sodium hypochlorite at pH 12.6 at 37°C for 7 days produced only log10 reductions ranging from 1.48 to 2 log10 (Shokraneh et al., 2014).
Summarised data from the references identified in the literature review for E. faecalis are displayed in Table 6.
3.7.2. Salmonella Senftenberg
Chemical inactivation of Salmonella Senftenberg was evaluated in liquid media of low pH (apple and orange juice) (Alvarez‐Ordóñez et al., 2009) or in sludge or dewatered pig and poultry manures (Paluszak et al., 2006; EFSA BIOHAZ Panel, 2010). In liquid media, D‐values varied between 12.2 and 54.9 minutes at pH 2.5, but as temperature increased D‐values were reduced down to a few seconds. As for Enterococcus faecalis, the lime‐treated dewatered pig and poultry manures (pH 12, 30 min at 70°C or 60 min at 60°C) following a thermo‐chemical treatment led to a 5 log10 reduction of S. Senftenberg (EFSA BIOHAZ Panel, 2010). In sludge, a 5 log10 reduction was achieved in 30 min with a quicklime (CaO) concentration of 20% and in 12 hours with 10% quicklime (Paluszak et al., 2006).
Summarised data from the references identified in the literature review for S. Senftenberg are displayed in Table 7.
3.7.3. Ascaris spp.
Inactivation of Ascaris spp. and Ascaris eggs has been mainly studied in sewage sludge, manure and similar materials increasing the pH > 12 by adding alkaline additives such as lime and quicklime in uncontrolled conditions of temperature and time (Table 8). Lime‐treated sludges can require several months until Ascaris eggs and adults are inactivated (Maya et al., 2010). However, the same treatment required much shorter time (even after 5 min) to observe inactivation at temperatures above 55°C (Capizzi‐Banas et al., 2004), leading to the conclusion that an increased temperature and pressure shortened the time needed for inactivation. Conflicting results exist regarding the combination of time and temperature needed to reach the level of inactivation of 3 log10 of Ascaris sp. in these types of matrices (Eriksen et al., 1996; Jimenez et al., 2000; Capizzi‐Banas et al., 2004; Paulsrud et al., 2004; Fitzmorris et al., 2007; EFSA BIOHAZ Panel, 2010), highlighting also the higher resistance of Ascaris eggs to inactivation, compared to larvae or adults.
Summarised data from the references identified in the literature review for Ascaris spp. are displayed in Table 8.
3.7.4. Viruses
3.7.4.1. Parvoviridae
A quicklime treatment inactivated > 3 log10 of Parvoviridae in dewatered pig and poultry manure in 60 or 30 min at a temperature of 60°C or 70°C, respectively (EFSA BIOHAZ Panel, 2010). The EFSA opinion does not specify the concentration of quicklime in the substrate of the assessed method but cites Ostertag (1987) recommending a dose of 200 g CaO/1,000 g of dry matter in sewage sludge, and states that ‘lime is also alkaline and a saturated solution (1.16 g/L) will impart a pH of 12.4 at 25°C’.
Full inactivation was observed in digested sludge after a lime treatment of 12 h (Strauch, 1983). In an acidic environment, the level of inactivation of Protoparvovirus was > 3 log10 at pH 1.7 in both buffer solution after 8 min and serum after 8 h (Yang et al., 2020).
Summarised data from the references identified in the literature review for Parvoviridae are displayed in Table 9.
3.7.4.2. Picornaviridae
Chemical inactivation of Picornaviridae in media other than sludge has been typically studied in acidic or near neutral pH and in most cases > 3 log10 of inactivation have been observed (Nims and Zhou, 2016) with short times (15 min) and at room temperature (20°C). Treating sludge with lime produced full inactivation (Goddard et al., 1982; Strauch, 1983).
Summarised data from the references identified in the literature review for Picornaviridae are displayed in Table 10.
3.7.4.3. Reoviridae
Chemical inactivation of Reoviridae has been studied in sewage, sludge or in porcine small intestine (Table 11). Reoviridae were fully inactivated in sewage sludge after treatment with quicklime (Strauch, 1983; Bosch et al., 1986), whereas in porcine small intestine 5.5 min were required to reduce the viral population by 1 log when treated with a weak acid (peracetic acid) and aqueous ethanol mixture (Hodde and Hiles, 2002).
Summarised data from the references identified in the literature review for Reoviridae are displayed in Table 11.
3.8. Integration of evidence on the efficacy of the processes to reduce biological hazards and indicator microorganisms
The current section integrates the available information retrieved on the thermal and chemical inactivation of the main biological hazards and indicator microorganisms to support the assessment made in the Expert Knowledge Elicitation (EKE) exercise on whether the processing standards for the declaration of the end points in the manufacturing chain or the standard or alternative methods approved for the production of derived products from the ABP in the list of materials achieve (i) a reduction of 5 log10 of Enterococcus faecalis or Salmonella Senftenberg (775W, H2S negative) and a reduction of infectivity titre by at least 3 log10 of those thermoresistant viruses that were identified as a relevant hazard (or of Parvoviridae, as a worse‐case scenario, when no intrinsic viral hazards were identified), and, in the case of chemical processes, also a reduction of eggs of Ascaris sp. by 3 log10 (Figure 10).
Figure 10.
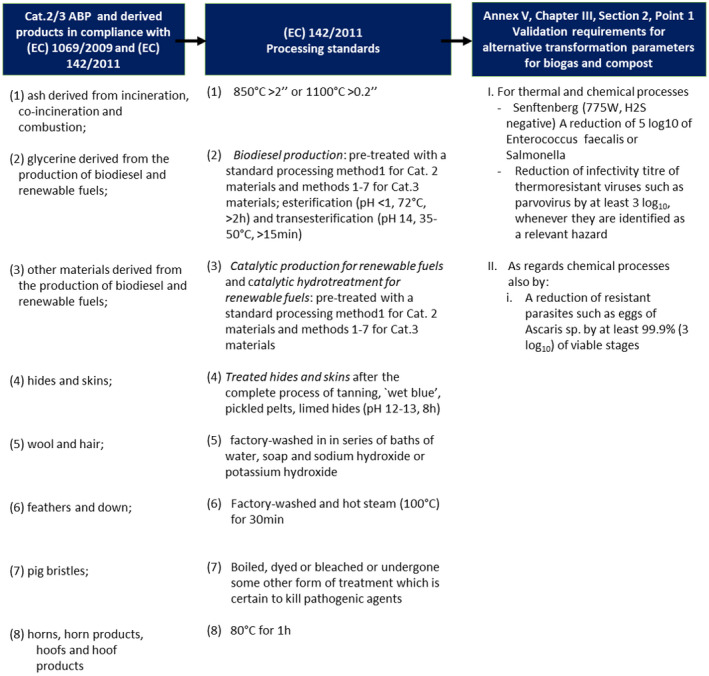
Components of the framework protocol applied to the assessment
3.8.1. Ash derived from incineration, co‐incineration and combustion
A wide range of biological hazards may occur in the ABP raw materials before their incineration, co‐incineration or combustion. However, bacteria, viruses and parasites are generally sensitive to heat and cannot survive normal burning temperatures. Prions are considered the most resistant biological hazards. Even the risk of TSE infectivity from ash would be extremely small if incineration is conducted at 850°C (SEAC, 2003). Indeed, the incineration at > 850°C is recognised in the EU as the standard method for disposing of waste.26 Therefore, ash derived from incineration, co‐incineration or combustion is typically considered safe and may be disposed of in landfills.
Ash generated from incineration, co‐incineration or combustion (carried out in accordance with Annex III of Commission Regulation (EU) No 142/2011) requires operating temperatures of 850°C for at least 2 seconds or 1,100°C for at least 0.2 s.
For Enterococcus faecalis, the collated data suggest that, for a time period of 2 s, greater than a 5 log10 reduction can be achieved at temperatures in excess of 98°C. Similarly, for a time period of 0.2 s then greater than a 5 log10 reduction can be achieved with a temperature of over 110.5°C. Both these temperatures (98°C and 110.5°C) are much lower than the operating temperatures of 850°C and 1,100°C (for the time intervals 2 and 0.2 s, respectively).
In order to achieve a 5 log10 reduction of S. Senftenberg 775W within a 2‐second time interval, the collated data suggest that temperatures of greater than 74°C would be sufficient. Similarly, to achieve a 5 log10 reduction of S. Senftenberg within a 0.2‐s time interval a temperature of greater than 78.8°C would be sufficient. Both temperatures (74°C and 78.8°C) are far below the temperatures experienced during thermal treatment by incineration, co‐incineration or combustion.
For viruses, from the data presented it is clear that at high process temperatures, the times required to achieve a 3 log10 reduction in the identified viruses are significantly reduced. Parvovirus is particularly resistant to high temperatures. However, multiple studies have shown a reduction of over 3 log10 of parvovirus within short time periods, for example, Nims and Plavsic (2013b) reported a 4 log10 reduction in parvovirus in 0.5 min at temperatures of 101, 112, 117°C in water, and 196°C in culture media.
3.8.2. Glycerine derived from the production of biodiesel and renewable fuels
A wide range of bacterial, viral, parasitic, protozoan and fungal pathogens can be found in the feedstock materials used for biodiesel production. However, the esterification and transesterification steps and the final vacuum distillation during biodiesel production have been considered to achieve a relevant reduction of biological hazards, including TSE agents, the most resilient pathogens (EFSA BIOHAZ Panel, 2015, 2017, 2020, 2021).
Biodiesel/glycerine production involves various thermal and chemical processes with capability to inactivate biological hazards. In the standard biodiesel production process, according to the EU legislation, the Cat. 2 or 3 ABP will be initially subjected to Method 1 (pressure sterilisation) in the case of Cat. 2 materials or methods 1–7 in the case of Cat. 3 materials, followed by an esterification (at pH < 1 by adding sulfuric acid or an equivalent acid and at 72°C for at least 2 h27), a transesterification (twice at pH ˜ 14 with potassium hydroxide or with an equivalent base at 35–50°C for at least 15 min), and a final distillation at 150°C, leading to the end‐product biodiesel and the co‐product glycerine.
The thermal processes to be considered are: Method 1 (133°C, 20 min 3 bar; if Cat. 2 material is used as feedstock for biodiesel production) or Method 5 (80°C 120 min and 100°C 60 min; if Cat. 3 material is used as feedstock for biodiesel production, method 5 being the least stringent in terms of temperature of the first five processing methods of ABP according to Commission Regulation (EU) 142/2001). These processes alone have the capacity to significantly reduce the population of both S. Senftenberg and E. faecalis. Thus, considering the data retrieved on the thermal resistance of these two microorganisms, short thermal treatments involving pasteurisation temperatures (< 100°C) resulted in D‐values (times required for a 1 log10 reduction in the bacterial population at a given temperature) in most of the matrices of < 1 min at temperatures ≥ 70°C. The exception was melted chocolate, a matrix known to have low water activity and high fat content, where S. Senftenberg showed D80 and D90‐values of 116 and 36 min, respectively (Doyle and Mazzotta, 2000). In addition, the subsequent esterification and transesterification processes, at 72°C and 35–50°C, respectively, and the final distillation at 150°C, as well as the extreme acid (pH < 1) and alkaline (pH ˜ 14) conditions prevailing during these processes will also contribute to lethality. Similarly, the harsh temperatures and pH conditions of the biodiesel/glycerine production process can also achieve a significant reduction of thermoresistant viruses, such as parvovirus. Thus, for example, a treatment for 0.5 min at 101°C produced a reduction of 4 log10 of bovine parvovirus in water in the study by Nims and Plavsic (2013b), and > 3.3 log10 reductions of porcine parvovirus have been obtained after treatments of 8 min at pH 1.7 in buffer solution supplemented with pepsin by Yang et al. (2020). Moreover, a quicklime (CaO) treatment of 30 min at 70°C and pH 12 caused a > 5 log10 reduction of S. Senftenberg and E. faecalis and a > 3 log10 reduction of parvovirus in dewatered pig (28.5% dry matter content) and poultry (40% dry matter content) manure (EFSA BIOHAZ Panel, 2010).
Likewise, other renewable fuels may be produced, following the EU legislation, through multistep catalytic processes involving a thermal treatment at very high temperatures (e.g. 250°C at 20 bar for at least 20 min; 265°C at 30 bar for at least 20 min).
It is worth highlighting that, as set out in Article 20 of Regulation (EC) No 1069/2009, alternative methods for the production of biodiesel and renewable fuels have been assessed by EFSA to ensure that any risks to public or animal health are reduced to a degree that is at least equivalent to that achieved by the processing methods that have already been approved for the same category of ABP. This is carried out by assessing whether the alternative methods achieve a relevant reduction, even for TSE agents, which are considered to be the most resistant biological hazards (EFSA BIOHAZ Panel, 2011, 2017, 2020, 2021).
3.8.3. Other materials derived from the production of biodiesel and renewable fuels
The main by‐products generated in the biodiesel and renewable fuels production processes are clay from bleaching and sludge from the pretreatment processes. These two by‐products are generated before some of the steps contributing lethality are applied in the production process (e.g. before the esterification, transesterification and distillation steps, or the multistep catalytic treatment), but after the raw materials are subjected to Method 1 (for Cat. 2 ABP) or Method 1–7 (for Cat. 3 ABP).
According to point D, Section 2, Chapter IV, Annex IV of Commission Regulation (EU) 142/2011 as amended, a fat fraction derived from ABP of all categories may be used for the production of biodiesel. Category 1 or Category 2 materials must be first processed using processing Method 1 (pressure sterilisation: 20 min, 133°C, 3 bar) as set out in Chapter III of the same Annex. According to point 2J and 2L, fish oil or rendered fats derived from Category 3 material can be used as a starting material, as long as they have been processed using any of the processing methods 1–5 or processing method 7; or in the case of material derived from fish oil, any of the processing methods 1–7.
As already mentioned, when dealing with the levels of reduction achieved throughout the production of glycerine, Method 1 and Method 5 have the capacity to inactivate S. Senftenberg, E. faecalis and parvovirus.
For Category 3 material subject to the least stringent treatment in terms of temperature as a worst‐case scenario (Method 5), the level of inactivation achieved for the relevant indicator microorganisms will be lower than for Category material 2, treated with Method 1. As previously described, the thermal resistance of these microorganisms will be high in matrices with low aw levels and high fat content (e.g. S. Senftenberg showed D80 and D90‐values in chocolate of 116 and 36 min, respectively (Doyle and Mazzotta, 2000) and E. faecalis showed D88 values of 0.36 min in raw almond kernels (Harris et al., 2012)). There are some reports showing a > 3 log10 reduction of parvovirus under similar time/temperature scenarios as those of Method 5 (e.g. a treatment for 0.5 min at 101°C produced a reduction of 4 log10 of Bovine parvovirus in water in the study by Nims and Plavsic (2013b)). On other occasions, a lower reduction level has been documented, e.g. a D80‐value of 23.6 min has been estimated for Bovine parvovirus based on the thermal inactivation data reported at different temperatures by Nims and Zhou (2016), which would result in a time of 70.8 min required at 80°C to reach a 3 log10 reduction of this viral indicator.
3.8.4. Hides and skins
In terms of the processes that may result in a reduction or elimination of indicator microorganisms during the treatment and post‐treatment (processing) of hides and skins, the first one to be assessed is the process for untreated hides and skins as described in the legislation and in Section 3.2.4, by applying one of the five available options. Excluding option (e) preservation process other than tanning, and (a) dried, option (d) allows the treatment of hides and skins by drying for a period of at least 42 days at a temperature of at least 20°C. This option would reduce the humidity of the raw material but would have uncertain, although limited, ability to reduce the indicator microorganisms. The other two options (b) and (c) include the addition of salt, and an extra alkaline additive in the case of (c). In both cases, a higher degree of inactivation is expected than for (a), (d) or (e).
Once the hides and skins are preserved, the steps that may result in a reduction or elimination of indicator microorganisms during the processing for the production of treated hides and skins to be placed in the market without restrictions include:
-
Limed hides:
-
–
the physical removal of blood, flesh, fat and hair including the full epidermis
-
–
the chemical liming treatments, which include the immersion in brine of lime at a pH 12–13 for at least 8 h.
-
–
-
Pickled pelts:
-
–
the physical removal of blood, flesh, fat and hair including the full epidermis
-
–
the chemical treatments, which include multiple immersion in alkaline solutions of pH > 8.5 and pH > 12 for variable time periods, usually several hours, followed by acidic solutions at pH < 3, also for several hours.
-
–
the splitting, which can separate the grain, i.e. the external layer above the corium, from the grain and corium junction and the corium, which results in what is also called by the leather industry as split leather.
-
–
bactericides and fungicides can be added to the resulting material, the pickled pelt.
-
–
-
‘Wet blue’:
-
–
there is no difference compared to the pickled pelts because the addition of chromium salts and alkaline buffers do not change the conditions of the material to produce further reduction of the indicator microorganisms or their elimination if this has not occurred yet during pickling.
-
–
-
Hides and skins that have undergone the complete process of tanning:
-
–
there is no difference compared to the pickled pelts or the wet blues because the addition of tanning agents, the removal of fat, the drying and the coating are not expected to contribute to a further reduction of the indicator microorganisms or their elimination if this has not occurred yet during pickling and wetting.
-
–
Hides and skins are the first group of materials included in the mandate that are subject only to chemical treatment. The different steps of the pretreatment cause the physical removal of tissues not intrinsic to the raw material that could contain indicator microorganisms or parasites: fat, blood, connective tissue, etc. However, the most important factor associated with hazard reduction is the alkaline treatment for ‘limed hides’ at pH 12–13 for 8 h and the subsequent acidic treatment of pH < 3 for circa 16 h for ‘pickled pelts’, ‘wet blue’ and ‘hides/skins undergoing complete tanning.
Enterococcus faecalis is a well‐known thermoresistant non‐spore‐forming bacterium that also has a high pH tolerance (Shokraneh et al., 2014), being able to grow over a wide range of pH: 4–11 (Nakajo et al., 2005). The matrix considerably influences the level of inactivation of E. faecalis. For example, in dewatered pig manure with a total solid content of 28.5% and maximum particle size of 12 mm a treatment of quicklime (CaO) at 70°C and pH > 12 achieved the 5 log10 reduction in a matter of 30 min (EFSA BIOHAZ Panel, 2010). The same level of inactivation was achieved for S. Senftenberg, and the required 3 log10 for parvovirus and Ascaris eggs. At lower temperature, the same treatment in horse manure and soil mixtures required up to 48 hours to achieve the 5 log10 reduction of E. faecalis (Nyberg et al., 2011).
Despite having less data, similar behaviour has been observed with S. Senftenberg, also known to be thermoresistant and to be able to survive in acidic environments. In a set of experiments in liquid matrices (fruit juices) with a pH adjusted to 2.5 at 37°C S. enterica serovar Senftenberg CECT 4348 showed D‐values ranging between 12.2 and 54.9 min, depending on the matrix (Álvarez‐Ordóñez et al., 2009).
In addition to the reduction caused by alkaline and acidic treatments, the salt applied to hides and skins as a preservative before starting the treatment process can also cause bacterial death due to osmotic shock (Davidson and Taylor, 2001) or retarded growth due to the reduction of aw in the matrix and the limited oxygen availability interfering with cellular enzymes (Shelef and Seiter, 2005).
The viral hazards identified in hides and skins were: Papillomaviridae (bovine papillomavirus, cottontail rabbit papillomavirus), Reoviridae (bluetongue virus), and Picornaviridae (FMDV, SVDV).
Starting with Picornaviridae, multiple viruses of this family (human enterovirus, FMDV, Equine rhinovirus) were reduced by > 3 log10 in cell cultures at 20°C with pH values of 3, 4 and 5 in 15 min whereas other viruses of the same family (Cardiovirus, porcine teschovirus, bovine and other enterovirus) did not reach such levels of reduction under similar conditions (Nims and Zhou, 2016). In a different substrate with an alkaline treatment, poliovirus in raw sewage sludge was fully inactivated in 30 min at pH 12 with a treatment of 3 kg/m3 calcium oxide (CaO), and in 360 min in digested sludge under the same treatment, while reovirus was inactivated at the same level after 180 min (Koch and Strauch, 1981). Mixed raw and humus sludge subject to filter pressing and liming at pH 10–11 achieved a full inactivation of enteroviruses, with the authors arguing that the high pH was enough to inactivate poliovirus and reovirus (Goddard et al., 1982). The same results were reported by Bosch et al. (1986) in raw sludge under the same treatment for 30 min at pH = 12. However, in these studies from the early 80s, there was no measurement of the reduction of the virus due to the difficulty of homogenising the raw material and measuring the initial viral load. No data were available on the chemical inactivation of papillomaviruses.
For chemical treatment only, the requirements for alternative transformation parameters for biogas and composting also include the demonstration of a reduction of resistant parasites such as eggs of Ascaris sp. by at least 99.9% (3 log10) of viable stages.
Ascaris are helminths known for their resistance to extreme conditions; hence, their use as an indicator for effective treatment of biosolids, sewage sludge, etc. Ascaris eggs may survive within a wide range of temperature (40–108°C), humidity (5–55%) and pH (9–13) (Maya et al., 2010), due to multiple layers acting as a natural barrier. Most of the treatments applied to the substrates as described in Table 8 are applied in aerobic conditions and at room temperature even though the chemical reactions after the application of chemicals like quicklime, slaked lime, etc., liberate heat, increasing the temperature and the dry solid content due to evaporation. In general, in matrices with total solid contents of 20% or lower, in which the pH has been increased to 12 or above with the consequent increase in temperature, all adult worms and larvae would be totally inactivated after a short period of time (less than 1 h). For example, quicklime (pH > 12) reaching 55°C achieved a 2.7 log10 reduction of viable Ascaris in dewatered sludge in 40 min and 3–3.4 log10 reductions in aerobic sludge under the same conditions (Brisolara and Reimers, 2013).
Ascaris eggs, however, are more resistant. If the humidity is high and the temperature equals room temperature, the time required to achieve the 3 log10 reduction of Ascaris eggs will be longer, in some cases, up to several days. For example, addition of 20% CaO to sludge would kill all Ascaris suum eggs after 6 hours (Paluszak et al., 2006), while quicklime at 60°C and pH of 12 in dewatered pig and poultry manure would achieve a 3 log10 reduction in Ascaris eggs after 60 min.
3.8.5. Wool and hair
The hydrolysis of wool with superheated water in a laboratory scale microwave reactor at different temperatures was studied by Zoccola et al. (2015) and optimised at 170°C for 60 min with a solid to liquor ratio close to 1.
No specific data are available in the literature regarding the duration of factory washing processes for wool and hair. Nonetheless, according to the sparse information available, it is performed with solutions with pH values > 12–13. Alkaline hydrolysis is performed with sodium hydroxide or potassium hydroxide characterised by pH values > 12. According to the literature available for wool and hair, alkaline hydrolysis is often associated with a thermal treatment at 120–170°C for 20–60 min. Since the papers reported above (Section 3.2.5) indicate a time period for the duration of alkaline hydrolysis ranging between 20 and 60 min, the WG considered the following time scenarios for the evaluation of the efficacy of the treatments: 5 min (as worst‐case scenario) and 60 min.
There are few data available on the inactivation of S. Senftenberg and E. faecalis by alkaline treatments. However, a 30‐min treatment with quicklime at 70°C and pH > 12 achieved, in dewatered pig manure with a total solid content of 28.5% and maximum particle size of 12 mm, a 5 log10 reduction for both indicator microorganisms (EFSA BIOHAZ Panel, 2010). Table 4 shows that the viruses identified as relevant hazards in wool and hair are Picornaviridae (FMDV) and Parvoviridae (Ungulate tetraparvovirus). No specific data are available on the inactivation of FMDV at pH 12 but for Picornaviridae Koch and Strauch (1981) demonstrated the inactivation of poliovirus in raw sludge after a treatment of 30 min with 3 kg/m3 CaO.
According to Capizzi‐Banas et al. (2004), no viable eggs of Ascaris sp. can be detected in sewage sludge at pH > 12 after a treatment of 5 min at 58°C. A 30‐min treatment with quicklime (CaO) at 70°C and pH > 12 achieved, in dewatered pig manure with a total solid content of 28.5% and maximum particle size of 12 mm, a 3 log10 reduction of parvovirus and Ascaris spp. (EFSA BIOHAZ Panel, 2010).
3.8.6. Feathers and down
The treatment of feathers and down can include (1) washing/degreasing and (2) thermal treatment with hot steam at 100°C for at least 30 min. As previously mentioned, considering the data retrieved on the thermal resistance of S. Senftenberg and E. faecalis, short thermal treatments involving pasteurisation temperatures (< 100°C) would be sufficient to achieve a 5 log10 reduction of these two indicator microorganisms, which show D‐values in most of the matrices of < 1 min at temperatures ≥ 70 min. For example, Saucier and Plamondon (2011) demonstrated that Enterococcus faecalis ATCC 7080 cultivated in BHI and ME2 media, respectively, spiked in ground beef and then treated in a water bath at 70°C displayed mean D‐values of 0.19 min and 0.15 min, respectively. Doyle and Mazzotta (2000) reported that S. Senftenberg in ground beef heated to 68°C displayed a mean D‐value of 0.22 min. Exceptions include those matrices with low water activity and high fat content, such as melted chocolate, where S. Senftenberg showed D80‐valuesand D90‐values of 116 min and 36 min, respectively (Doyle and Mazzotta, 2000). However, the washing and thermal treatment with steam will add moisture to the raw material, thus favouring the inactivation, as compared to a totally dried matrix or the application of dry heat.
Table 4 summarises the viruses which have been identified as relevant hazards in feathers and down. The most thermoresistant, represented by the non‐enveloped DNA viruses, were CAV belonging to the family Anelloviridae, and Duck circovirus, belonging to the family Circoviridae.
According to Urlings et al. (1993), a 5 log10 reduction was achieved, after a treatment of 100°C for 10 min or 95°C for 30 min, for CAV, which was not detected in minced meat from chicken carcasses experimentally infected with the virus after heating in a stirred water bath. The same result was obtained when testing minced meat supplemented with 4% w/w dextrose heated in a stirred water bath. Welch et al. (2006) quantified a 1 log10 reduction in the level of CAV in human serum albumin after a dry heat treatment at 120°C for 30 min. Finally, in chicken by‐products, CAV was inactivated at 100°C after 10 min (EFSA AHAW Panel and EFSA BIOHAZ Panel, 2011). No data were available on the thermal inactivation of Duck circovirus. The data extracted exclusively included thermal reduction data for porcine circovirus and showed that treatments of 30 min at 75°C, or 30 min at 120°C (with dry heat) produce log reductions < 3 log10 (Welch et al., 2006).
3.8.7. Pig bristles
For pig bristles, the standard against which the level of inactivation of indicator microorganisms was assessed was aboiling, i.e. immersion of the raw material in water at no less than 100°C for a determined period of time. Given the absence of any minimum time specified in the legislation, it was agreed to assess two scenarios for different times, 5 min (as worst‐case scenario) and 60 min, for the evaluation of the efficacy of the treatments.
According to the thermal inactivation data gathered and described in Section 3.6 for S. Senftenberg, most of the data confirmed that the 5 log10 can be achieved in a very short time (< 5 min) at temperatures of 70°C or above. The only data points which are well above the two time/temperature combinations under assessment are those measured in matrices with high fat content (melted chocolate). For E. faecalis, there are no data points in which the 5 log10 level of inactivation required longer than the shortest time under assessment, i.e. 5 min.
No virus was identified intrinsically in pig bristles during the virial hazard identification. Thus, the Parvoviridae family was used as the indicator virus against which to assess the efficacy of the processing method. Despite the high heat resistance of this virus, the conditions of boiling in water at 100°C seem to cause a significant reduction of parvovirus. At 103°C, Lelie et al. (1987) reported total inactivation of Canine parvovirus in human serum protein solution in 1.5 min. In a similar substrate, human plasma, 300 min was needed to reduce 4 log10 applying dry heat at 100°C (Bräuniger et al., 2000). In water, a 1 log10 reduction occurred in 0.5 min at 94°C for Bovine parvovirus, needing 101°C to achieve a 4 log10 reduction in the same time (Nims and Plavsic, 2013b). In matrices with more solid content, like manure mixed with bleaching clay, 660 min was needed to achieve 4 log10 reduction of porcine parvovirus at 55°C (Lund et al., 1996), and 1,019 min to reduce 1 log10 of porcine parvovirus at 49°C in bovine faeces (Elving et al., 2014). Sauerbrei and Wutzler (2009), cited by EFSA BIOHAZ Panel (2011), concluded that Bovine parvovirus was not significantly influenced by dry heat at 95°C for 120 min.
As the papers reported above (Section 3.2.7) indicate a time period for the duration of the alkaline hydrolysis ranging between 20 and 60 min, the WG considered the following time scenarios for the evaluation of the efficacy of the treatments: 5 min (as worst‐case scenario) and 60 min.
3.8.8. Horns, horn products, hooves and hoof products
According to the legislation, for the placing on the market of horns and horn products, excluding horn meal, and hooves and hoof products, excluding hoof meal, intended for the production of organic fertilisers or soil improvers, they shall be subject to a heat treatment for 1 hour at a core temperature of at least 80°C.
As previously mentioned, considering the data retrieved on the thermal resistance of S. Senftenberg and E. faecalis, short thermal treatments involving pasteurisation temperatures (< 100°C) showed D‐values in most of the matrices of < 1 min at temperatures ≥ 70°C. The exception are those matrices with low aw and high fat content, such as melted chocolate, where S. Senftenberg showed D80‐value and D90‐value of 116 min and 36 min, respectively, in experiments conducted with cells grown to stationary phase and then inoculated into melted chocolate and heated to the target temperature (Doyle and Mazzotta, 2000).
In terms of virus, Table 4 shows that the virus identified as a relevant hazard for hooves and horns is Senecavirus (Picornaviridae). According to literature search results reported in Section 3.6, specific data on thermal inactivation were only available for ‘swine vesicular disease’, not for ‘Senecavirus A’. However, data were retrieved on thermal inactivation of other Picornaviridae family members (Table A.5). El‐Senousy et al. (2020) reported 1.2 log10 reductions of human hepatitis A virus (Picornaviridae) after 2 min at 80°C. Other authors (Knight et al., 2013) reviewed the thermal inactivation of animal virus pathogens including Picornaviridae, indicating for small ssRNA non‐enveloped viruses D‐values of 10 s at a temperature of 70–74°C.
4. Uncertainty analysis
Table 12.
Sources of uncertainty associated with the AQs and their possible impact on the conclusions
| Source of uncertainty | Cause of the uncertainty | Impact of the uncertainty on the conclusions |
|---|---|---|
| Ascertainment of the standard processes for hides and skins | The treatments applied to hides and skins for three of the four types of products declared end points were ascertained by searching in the scientific literature and from industry information in grey literature. The steps and conditions of the treatments could vary except for the lime hides which are specified in the legislation | If the actual treatments applied for the production of pickled pelts, wet blues and tanned hides and skins are different than those described in Section 3, this could result in the higher or lower inactivation of the indicator microorganisms and biological hazards. Including pretreatment may result in greater inactivation |
| Treatment of hides and skins before processing | The hides and skins before going into tanning must be treated according to Commission Regulation (EU) No 142/2011, which means they are (a) dried; (b) dry‐salted or wet‐salted for a period of at least 14 days before dispatch; (c) salted for a period of at least 7 days in sea salt with the addition of 2% of sodium carbonate; (d) dried for a period of at least 42 days at a temperature of at least 20°C; or (e) subject to a preservation process other than tanning. The potential reduction of indicator microorganisms during this treatment has not been considered | It is expected that some level of reduction will occur due to the treatment of hides and skins, resulting in a higher reduction of the indicator microorganisms in the entire process (treatment and processing of the four conditions for the declaration of end points) |
| Standard or alternative methods for the production of derived products using Category 3 materials | The methods to be applied to Category 3 materials in the pre‐processing of fat for the production of biodiesel and renewable fuels range from Method 1 to Method 7, which have very different parameters in terms of temperature and time combinations. Method 7 requires microbiological criteria ensuring the absence of Salmonella and Clostridium perfringens, and certain level of Enterococcus spp. Method 5 is the least stringent in terms of temperature of those with explicit parameters in terms of temperature, while Method 6 only applies to aquatic animals/aquatic invertebrates. It was decided to apply as worst‐case scenario the least stringent method in terms of temperature (i.e. Method 5) | Depending on the method applied to the raw Category 3 material, the inactivation of the indicator microorganisms in the raw material could be higher than the estimated inactivation on the basis of the conditions given for Method 5, except for Method 7, which could result in a higher or lower inactivation level |
| Ascertainment of the transformation processes for the declaration of the end points for wool and hair | Commission Regulation (EU) No 142/2011 does not identify specific technical parameters for the declaration of the end point for wool and hair, and the information retrieved from the literature on the processing of these ABP was scarce. Based on the background information two scenarios were selected to be assessed (pH > 12, 5 min; pH > 12, 60 min). There is the possibility that these two scenarios do not fully reflect current industrial practices | This may result in an over‐ or underestimation of the level of hazard reduction achieved |
| Ascertainment of the processes for pig bristles | Commission Regulation (EU) 142/2011 does not identify specific technical parameters for the declaration of the end point of pig bristles, and the information retrieved from the literature on the processing of these ABP materials was scarce. Based on the background information, two scenarios were selected for being assessed (boiling at 100°C for 5 or 60 min). There is the possibility that these two scenarios do not fully reflect current industrial practices | This may result in an over‐ or underestimation of the level of hazard reduction achieved |
| Nature of the chemical processes | Some of the processes under assessment based on chemical inactivation (e.g. liming) are exothermic processes which release heat causing a progressive increase in temperature in an uncontrolled manner. Therefore, the inactivation level attained in these processes may derive from combined thermo‐chemical effects | This may result in the overestimation of the level of inactivation of indicator microorganisms achieved by the chemical processes alone, due to the combination of both thermal and chemical effects |
| Identification of viral hazards | The viral hazards that may occur in the ABP listed in the mandate were identified through literature searches using search strings, as described in the data and methodologies section. There is the possibility that some relevant reference was not identified or considered. It is also possible that the occurrence of virus in general, or certain families, in particular, have never been investigated | As a result, it could be the case that some relevant virus was overlooked. This could impact on the conclusions only if some relevant heat‐ or chemical‐resistant virus (e.g. non‐enveloped viruses) was overlooked |
| Inactivation data | Relevant references for extraction of data on thermal and chemical reduction/inactivation of indicator microorganisms were identified through literature searches using search strings, as described in the data and methodologies section. There is the possibility that some relevant reference was not identified or considered for data extraction | This source of uncertainty could lead to either higher or lower inactivation of indicator microorganisms |
| Inactivation data | The data extracted on thermal and chemical reduction/inactivation of indicator microorganisms were sourced from experimental studies using different matrices to those included in the mandate. There are no specific data available from studies involving spiking the materials included in the mandate with the indicator microorganisms of interest. The different composition in terms of dry matter (total solid contents, aw), fat content, etc. determines the capacity of bacteria, viruses and parasites to survive under different conditions of temperature, time and pH | The capacity of the standard processes to achieve the targeted reductions may be higher or lower than estimated in the materials included in the mandate. In general, the materials have a low water content, while most inactivation data retrieved from the literature derive from studies using liquid media or foods. As microbial inactivation by heat and chemical processes is lower in systems with low water activity, estimations from studies on liquid media or on foods with high water activity could result in an overestimation of the inactivation of the group of materials included in the mandate by the transformation processes |
| Inactivation data | The data extracted on thermal and chemical reduction/inactivation of indicator microorganisms were sourced from experimental studies using particular strains/isolates of the relevant hazards and different analytical methods, which, for viral hazards, are not standardised. It is uncertain whether they are representative of the behaviour of the whole species | This source of uncertainty could lead to either higher or lower inactivation of the viral hazards |
| Inactivation data | The data retrieved on thermal inactivation of indicator microorganisms contained information on certain heating temperatures, that in some cases were far from the temperatures under assessment. An extrapolation to temperatures much higher than those used to calculate D‐ and Z‐values might not be accurate. The data extracted on chemical reduction/inactivation of indicator microorganisms were sourced from experimental studies using pH values and concentrations of agents in most cases different from those of the processing methods under assessment, and it is not possible from the available data to calculate equivalent inactivation levels at different pH values and concentrations of agents | This source of uncertainty could lead to either higher or lower inactivation predicted for the indicator microorganisms |
| Inactivation data | Some of the references retrieved lacked details on the initial load and/or the level of inactivation of the hazard. The data extracted were expressed as presented by the authors without any quantitative presentation or inclusion in the corresponding graph | This could lead to an under‐ or overestimation of the level of reduction achieved by the treatments described in those papers |
5. Answers to AQ 2, 4 and 5, obtained by expert knowledge elicitation
Following the procedure outlined in Section 2 and Annex A, an EKE was performed to answer AQ2, AQ4 and AQ5. The experts were requested to express the uncertainty about their judgements; that is how sure they were that the specified log10 reductions are achieved. In line with the EFSA's Guidance on Uncertainty Analysis in Scientific Assessment (EFSA Scientific Committee, 2018a), these uncertainties were expressed quantitatively, as probabilities. Potential variability in performance of the processes is addressed by asking whether specified reductions are obtained in more than 99% of cases, it is not included in the probability estimates given by the experts.
The following EKE questions were answered for each of the transformation processes for the declaration of the end points in the manufacturing chain (for raw materials) and the approved standard or alternative methods (for derived products), described for the different materials as in the answer to AQ1:
What is the probability that a 5 log10 reduction of E. faecalis is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 5 log10 reduction of Salmonella Senftenberg (775 W, H2S negative) is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 3 log10 reduction of parvovirus or the identified most resistant viruses is achieved, in more than 99% of cases, by application of the relevant process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
What is the probability that a 3 log10 reduction of eggs of Ascaris sp. is achieved, in more than 99% of cases, by application of the relevant chemical process/es, assuming that the process/es is/are performed as prescribed and that the indicated process conditions are achieved?
Expert judgements were obtained for three or four indicators in 16 combinations of transformation processes in the eight groups of materials considered, yielding a total of 52 combinations for which probability ranges, expressing the certainty that the indicated reductions are achieved, were produced.
As explained in Appendix C (EKE report), the first step of the EKE consisted of the individual judgements of the eight experts participating in the EKE, based on the evidence presented in this scientific opinion, the Uncertainty Table and their individual expertise. For most combinations, the judgements of the experts differed considerably (see Appendix C).
In Step 2 of the EKE, the experts met and agreed on a consensus judgement for all 52 combinations, based on their individual judgements and an exchange of arguments. These consensus judgements provided a probability range for each of the 52 combinations, which reflects the uncertainty of the experts and expresses the likelihood that the log reductions described in the Regulations are achieved. The results of the consensus EKE are given in Table 13 and Figure 11.
Table 13.
Material/process/hazard combinations included in the EKE and results of the consensus judgement
| Material | Transformation processes for the declaration of the end points and approved standard or alternative methods to produce derived products | Treatment | Indicator microorganism and required reduction | |||
|---|---|---|---|---|---|---|
| S. Senftenberg (5 log10) | E. faecalis (5 log10) | Ascaris eggs (3 log10) | Parvovirus (or virus/viruses as in the hazard identification) (3 log10) | |||
| 1) Ash derived from incineration | Parvoviridae | |||||
| 1.1. 850°C >2 s | T | 99–100% | 99–100% | 99–100% | ||
| 1.2. 1,100°C > 0.2 s | T | 99–100% | 99–100% | 99–100% | ||
| 2) Glycerine derived from the production of biodiesel and renewable fuels | Parvoviridae | |||||
| If Category 2 materials are used: Method 1 + esterification + transesterification | 2.1. 133°C, 20min 3 bar (Method 1) + pH < 1/72°C/> 2 h (esterification) + pH ˜ 14/35°C to 50°C/> 1 min (transesterification) | T/C | 98–100% | 98–100% | 98–100% | |
| If Category 3 materials are used: Method 1–7 + transesterification | 2.2. 80°C 120min (Method 5(a)*) + pH ˜ 14/35°C to 50°C/> 15 min (transesterification) | T/C | 90–99% | 90–99% | 90–95% | |
| If Category 3 materials are used: Method 1–7 + transesterification | 2.3. 100°C 60min (Method 5(b)*) + pH˜14/35°C to 50°C/> 15 min (transesterification) | T/C | 95–99% | 95–99% | 90–99% | |
| 3) Other products of materials derived from the production of biodiesel and renewable fuels | Parvoviridae | |||||
| If Category 2 materials are used: Method 1 | 3.1 Method 1: 133°C, 20 min 3 bar | T | 90–99% | 90–99% | 90–99% | |
| If Category 3 materials are used: Method 1–7 | 3.2 Method 5(a): 80°C, 120 min | T | 50–90% | 66–95% | 33–90% | |
| If Category 3 materials are used: Method 1–7 | 3.3 Method 5(b): 100°C, 60 min | T | 66–90% | 66–95% | 66–90% | |
| 4) Hides and skins | Papillomaviridae, Picornaviridae, Reoviridae | |||||
| Lime hides | 4.1. pH 12–13, 8 h | C | 66–90% | 66–90% | 10–66% | 33–66% |
| Pickled pelts | 4.2. pH ˜12 > 8 h + pH < 3, 16 h | C | 66–95% | 66–95% | 33–66% | 50–90% |
| Wet blue | pH ˜12 > 8 h + pH < 3, 16 h | |||||
| Complete tanned hides | pH ˜12 > 8 h + pH < 3, 16 h | |||||
| 5) Wool and hair | Picornaviridae, Parvoviridae | |||||
| Factory‐washing: immersion of the wool and hair in series of baths of water, soap and sodium hydroxide or potassium hydroxide | 5.1. pH > 12–13, 5 min | C | 10–50% | 10–50% | 1–33% | 10–50% |
| 5.2. pH > 12–13, 60 min | C | 33–80% | 33–80% | 10–50% | 33–66% | |
| 6) Feathers and down | Anelloviridae, Circoviridae | |||||
| Factory‐washed and treated with hot steam | 6. 100°C for at least 30 min | T | 90–99% | 90–99% | 66–90% | |
| 7) Pig bristles | Parvoviridae | |||||
| Boiling (from ASF countries) | 7.1. 100°C in water, 5 min | T | 80–95% | 80–95% | 33–66% | |
| 7.2. 100°C in water, 60 min | T | 95–99% | 95–99% | 50–95% | ||
| 8) Horns, horn products, hooves and hoof products | Picornaviridae | |||||
| Heat treatment | 8. 80°C, 1 h | T | 66–95% | 66–95% | 66–99% | |
*: Despite Method 5 must ensure that a core temperature greater than 80?C is achieved for at least 120 minutes and a core temperature greater than 100?C is achieved for at least 60 min, the assessment was conducted separate for the two temperature/time combinations. Note: Cells in grey, not applicable.
Figure 11.
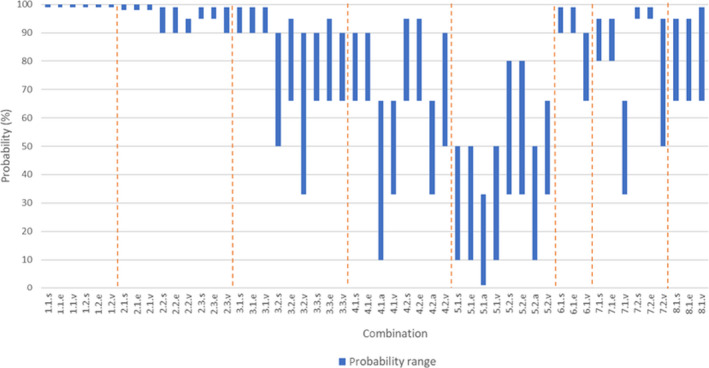
- Combinations are indicated as material number.Process number.Indicator (s: S. Senftenberg, e: E. faecalis; a: Ascaris eggs; v = virus).
6. Conclusions
Three types of materials were evaluated in this mandate as follows: (a) derived products after approved standard or alternative transformation processes are applied, as per Commission Regulation (EU) No 142/2011, to Category 2 and Category 3 ABP (Group 1), fat fractions of ABP (Group 2) or rendered fats (Group 3); (b) materials for which Commission Regulation (EU) 142/2011 establishes specific technical parameters of the transformation processes for the declaration of the end points in the manufacturing chain (Groups 4, 5 and 6); and (c) materials for which Commission Regulation (EU) 142/2011 establishes technical parameters of the transformation processes for the placement in the market (Groups 7 and 8).
It was assessed whether the technical parameters identified or selected in AQ1 meet the requirements for alternative transformation parameters for biogas and composting plants, referred to in point 1 of Section 2 of Chapter III of Annex V to Regulation (EU) No 142/2011. For Groups 5 and 7, Commission Regulation (EU) No 142/2011 does not provide specific technical parameters, such as time, for the transformation processes. Consequently, the approach followed was to assess whether the requirements are met in two different time scenarios: 5 min and 60 min. For Groups 2 and 3, where the standard processing methods of ABP applied for Category 3 ABP are not specified in Commission Regulation (EU) No 142/2011, the two combinations of time and temperature of Method 5 (the one of the first five methods at the lowest temperature) were considered in the assessment separately, despite the legal requirement of the two combinations to be achieved. In the final answer, the overall judgment for the entire process was made. The impact of the particle size was not taken into account.
-
For all groups, it was assessed whether the transformation processes can achieve a reduction of 5 log10 of E. faecalis or S. Senftenberg (775W, H2S negative) (AQ2) and a reduction of at least 3 log10 of parvovirus or thermoresistant viruses identified as relevant hazards for the specific materials (AQ4). For processes involving a chemical treatment, the reduction of eggs of Ascaris sp. by 3 log10 was also assessed (AQ5). The thermoresistant non‐enveloped viruses identified as relevant hazards were (AQ3):
-
–
Group 4 (Hides and skins): Papillomaviridae (Bovine papillomavirus, Cottontail rabbit papillomavirus), Reoviridae (Bluetongue virus) and Picornaviridae (Foot and mouth disease virus, Swine vesicular disease virus)
-
–
Group 5 (Wool and hair): Picornaviridae (Foot and mouth disease virus), and Parvoviridae (Ungulate tetraparvovirus)
-
–
Group 6 (Feathers and down): Anelloviridae (Chicken anaemia virus) and Circoviridae (Duck circovirus)
-
–
Group 8 (Horns, horn products, hooves and hoof products): Picornaviridae (Senecavirus A)
-
–
For the other groups of ABP (Groups 1, 2, 3 and 7), no viral hazards were identified and Parvoviridae were used as indicators as a worst‐case scenario to assess the efficacy of the processes at inactivating viral hazards.
There is no evidence of the intrinsic presence of some of these microorganisms in the materials under assessment. They are indicators used for the approval of alternative methods for biogas and compost production.
An expert knowledge elicitation (EKE) was undertaken considering the available evidence, existing data gaps and uncertainties to provide the answer to AQ2, AQ4 and AQ5. The combinations of materials and processes were judged for each of the relevant indicator microorganisms and biological hazards. The probability range estimates obtained for the most resistant indicator microorganisms and biological hazards were selected as the worst‐case scenario to describe the uncertainty around the efficacy of the transformation processes (i.e. the required log10 reduction is achieved in at least 99% of cases).
-
The probability that these processes achieve the required level of inactivation is always higher for other than the most resistant indicator microorganisms and biological hazards in each combination of material and treatment. Following this criterion, the EKE led to the following conclusions:
-
1
Ash derived from incineration, co‐incineration and combustion:
-
1
-
–
It was judged 99–100% certain that the transformation processes, as defined in the legislation (850°C, >2 s; 1,100°C, >0.2 s), are able to reduce, to the required extent, the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
-
2
Glycerine derived from the production of biodiesel and renewable fuels:
-
2
-
–
For Category 2 ABP, it was judged 98–100% certain that the transformation process, as defined in the legislation (Method 1 at 133°C, 20 min, 3 bar, followed by esterification and transesterification), is able to reduce, to the required extent, the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
-
–
For Category 3 ABP, it was judged 90–95% and 90–99% certain that the transformation processes, as defined in the legislation (80°C for 120 min and 100°C for 60 min, followed by transesterification), are able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae). Since method 5 must ensure that the two time–temperature combinations are met, even if they were assessed separately, it is considered at least 90–99% certain that the transformation process is able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
-
3
Other materials derived from the production of biodiesel and renewable fuels:
-
3
-
–
For Category 2 ABP, it was judged 90–99% certain that the transformation process, as defined in the legislation (Method 1 at 133°C, 20 min 3 bar), is able to reduce, to the required extent, the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
-
–
For Category 3 ABP, it was judged 33–90% and 66–90% certain that the transformation processes, as defined in the legislation (80°C for 120 min and 100°C for 60 min, respectively), are able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae). Since method 5 must ensure that the two time–temperature combinations are met, even if they were assessed separately, it is considered at least 66–90% certain that the transformation process is able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms (E. faecalis, S. Senftenberg and Parvoviridae).
-
4
Hides and skins:
-
4
-
–
It was judged 10–66% and 33–66% certain that the transformation processes, as defined in the legislation (pH 12–13 for 8h, and pH 12 for > 8 h followed by pH < 3 for 16 h, respectively), are able to reduce, to the required extent, eggs of Ascaris sp., the most resistant of the six biological hazards and indicator microorganisms (E. faecalis, S. Senftenberg, Papillomaviridae, Reoviridae, Picornaviridae and eggs of Ascaris sp.).
-
5
Wool and hair:
-
5
-
–
It was judged 1–33% and 10–50% certain that the transformation processes, as defined in the legislation (pH > 12–13), applied for 5 min or 60 min, respectively, are able to reduce, to the required extent, eggs of Ascaris sp., the most resistant of the five biological hazards and indicator microorganisms (E. faecalis, S. Senftenberg, Picornaviridae, Parvoviridae and eggs of Ascaris sp.).
-
–
The results of these two‐time scenarios revealed that the uncertainty for the efficacy of the transformation processes for wool and hair is very much dependent on the time of application. Longer times would result in the reduction of uncertainty on the efficacy of the process.
-
6
Feathers and down:
-
6
-
–
It was judged 66–90% certain that the transformation process, as defined in the legislation (100°C for at least 30 min), is able to reduce, to the required extent, Anelloviridae and Circoviridae, the most resistant of the four biological hazards and indicator microorganisms (E. faecalis, S. Senftenberg, Anelloviridae and Circoviridae).
-
7
Pig bristles:
-
7
-
–
It was judged 33–66% and 50–95% certain that the transformation process, as defined in the legislation (100°C), applied for 5 min or 60 min, respectively, is able to reduce, to the required extent, Parvoviridae, the most resistant of the three indicator microorganisms and biological hazards (E. faecalis, S. Senftenberg and Parvoviridae).
-
–
The results of these two‐time scenarios revealed that the uncertainty for the efficacy of the transformation processes for pig bristles is very much dependent on the time of application. Longer times would result in the reduction of the uncertainty on the efficacy of the process.
-
8
Horns, horn products, hooves and hoof products:
-
8
-
–
It was judged 66–95% certain that the transformation process, as defined in the legislation (80°C for 60 min), is able to reduce, to the required extent, the indicator bacteria (E. faecalis and S. Senftenberg), the most resistant of the three indicator microorganisms and biological hazards (E. faecalis, S. Senftenberg and Picornaviridae).
7. Recommendations
The data available on the intrinsic physicochemical properties (e.g. pH, aw) of most of the ABP under assessment, and on the occurrence of biological hazards in them, are very scarce. It is recommended to implement studies to fill these knowledge gaps.
Data available in the literature on thermal and non‐thermal (chemical) inactivation of indicator microorganisms and biological hazards are mainly derived from studies carried out in basic broth‐based laboratory models or food systems. It is recommended to undertake studies on the survival of biological hazards in ABP matrices, and if possible, in full‐scale systems, to support future risk assessments.
It is recommended to conduct a full characterisation of the usage pathways of ABP as OF/SI in the EU, to facilitate the development of future risk assessments.
The current opinion focusses on the question of whether the log10 reductions of indicator microorganisms and biological hazards, as given in the legislation, are achieved. The impact of these log10 reductions and the uncertainty on actual animal/public health risks has not been quantified. As a follow‐up, it is recommended that a risk assessment of the usage of ABP is considered, as this would provide insight into the consequences of deviations from the reductions that the processes should achieve. In the statement of purpose of such a risk assessment, the precise usage of the ABP should be clearly defined.
Glossary
Annex I of Commission Regulation (EU) No 142/2011 provides the following definitions relevant to this mandate, as referred to in Article 2. Definitions are presented by group of materials and the ones accompanied by * are derived from different sources.
- Processing methods
Methods listed in Chapters III and IV of Annex IV.
- By‐product*
An incidental or secondary product made in the manufacture or synthesis of a certain product.
- Co‐product*
A product with commercial relevance obtained during the manufacture or synthesis of another certain product, with common steps in the production process.
- Derived material*
Any material, different from the final product, obtained during a process of manufacture or synthesis.
- Group 1
- Incineration
The disposal of animal by‐products or derived products as waste, in an incineration plant, as defined in point 4 of Article 3 of Directive 2000/76/EC.
- Co‐incineration
The recovery or disposal of animal by‐products or derived products, if they are waste, in a co‐incineration plant.
- Combustion
A process involving the oxidisation of fuel in order to use the energy value of the animal by‐products or derived products, if they are not waste.
- Group 2–3
- Biodiesel*
Renewable fuel comprised of mono‐alkyl esters of long chain fatty acids derived from vegetable oils or animal fats.28
- Esterification*
The reaction between an alcohol (R‐COH) and a carboxylic acid (R’‐COOH) forming in the presence of a catalyst an ester (R‐COO-R’) and water (H2O). Typical alcohols used in esterification are methanol and ethanol. A reaction with free fatty acids results in fatty acid alkyl esters and water.29
- Glycerine*
C3H8O3, a co‐product from biodiesel production from animal by‐products (ABP) and vegetable oils.
- Insoluble impurities*
Solid material which remains non‐soluble in analytical solvent (commonly light petroleum) and can be isolated by filtration and weighed.
- Tallow*
Animal fat obtained after rendering of animal by‐products30
- Transesterification*
The reaction between an alcohol (R’’‐OH) and an ester (R‐COO-R’) forming in the presence of a catalyst a different ester (R‐COO-R’’) and a different alcohol (R’‐OH) with exchanged R groups. A reaction with triglycerides results in fatty acid alkyl esters and glycerol31
- Group 4
- Untreated hides and skins
All cutaneous and subcutaneous tissues that have not undergone any treatment, other than cutting, chilling or freezing.
- Treated hides and skins
Derived products from untreated hides and skins, other than dog chews, that have been: (a) dried; (b) dry‐salted or wet‐salted for a period of at least 14 days prior to dispatch; (c) salted for a period of at least seven days in sea salt with the addition of 2% of sodium carbonate; (d) dried for a period of at least 42 days at a temperature of at least 20°C; or (e) subject to a preservation process other than tanning.
- Quicklime
Calcium oxide, CaO. The reaction for the thermal decomposition of calcium carbonate and production of quicklime is described below.32 CaCO3 + heat = CaO + CO2.
- Slaked lime or hydrated lime
Calcium hydroxide, Ca(OH)2. Adding water to quicklime produces an exothermic reaction and hydrated lime, as described below.27 CaO + H2O = Ca(OH)2 + heat.
- Tanning
The hardening of hides, using vegetable tanning agents, chromium salts or other substances such as aluminium salts, ferric salts, silicic salts, aldehydes and quinones, or other synthetic hardening agents.
- Group 5
- Untreated wool
Wool, other than wool which has (a) undergone factory washing; (b) been obtained from tanning; or (c) been treated by another method that ensures that no unacceptable risks remain; (d) been produced from animals other than those of the porcine species, and has undergone factory‐washing consisting of the immersion of the wool in a series of baths of water, soap and sodium hydroxide or potassium hydroxide; or (e) been produced from animals other than those of the porcine species, is intended for being dispatched directly to a plant producing derived products from wool for the textile industry and has undergone at least one of the following treatments: (i) chemical depilation by means of slaked lime or sodium sulfide; (ii) fumigation in formaldehyde in a hermetically sealed chamber for at least 24 h; (iii) industrial scouring which consists of the immersion of wool in a water‐soluble detergent held at 60–70°C; (iv) storage, which may include the journey time, at 37°C for eight days, 18°C for 28 days or 4°C for 120 days.
- Untreated hair
Hair, other than hair which has (a) undergone factory washing; (b) been obtained from tanning; or (c) been treated by another method that ensures that no unacceptable risks remain; (d) been produced from animals other than those of the porcine species, and has undergone factory‐washing consisting of the immersion of the hair in a series of baths of water, soap and sodium hydroxide or potassium hydroxide; or (e) been produced from animals other than those of the porcine species, is intended for being dispatched directly to a plant producing derived products from hair for the textile industry and has undergone at least one of the following treatments: (i) chemical depilation by means of slaked lime or sodium sulfide; (ii) fumigation in formaldehyde in a hermetically sealed chamber for at least 24 h; (iii) industrial scouring which consists of the immersion of hair in a water‐soluble detergent held at 60–70°C; (iv) storage, which may include the journey time, at 37°C for eight days, 18°C for 28 days or 4°C for 120 days.
- Group 6
- Untreated feathers and parts of feathers
Feathers and parts of feathers, other than feathers or parts of feathers which have been treated (a) with a steam current or (b) by another method that ensures that no unacceptable risks remain.
- Group 7
- Untreated pig bristles
Pig bristles, other than pig bristles which have (a) undergone factory washing; (b) been obtained from tanning; or (c) been treated by another method that ensures that no unacceptable risks remain.
Abbreviations and acronyms
- ABP
Animal by‐product
- AEV
Avian encephalomyelitis virus
- AQ
Assessment question
- ASF
African swine fever
- aw
Water activity
- BIOHAZ Panel
EFSA Panel on Biological Hazards
- BSE
Bovine spongiform encephalopathy
- CAV
Chicken anaemia virus
- CFU
Colony forming unit
- EF
Enterococcus faecalis
- EKE
Expert Knowledge Elicitation
- ELS
Extensive literature search
- FFA
Free fatty acids
- FMD
Foot-and‐mouth disease
- FMDV
Foot-and‐mouth disease virus
- MVM
Minute virus of mice
- n/a
Not applicable
- OF/SI
Organic fertilisers and soil improvers
- PFU
Plaque‐forming unit
- RIO
Rational impartial observer
- SSC
Scientific Steering Committee
- SVD
Swine vesicular disease
- SVDV
SVD virus
- ToR
Terms of Reference
- TSE
Transmissible spongiform encephalopathy
- WG
Working Group
Appendix A – Thermal inactivation data
1.
Appendix B – Processing methods of ABP according to Commission Regulation (EU) No 142/2011
1.
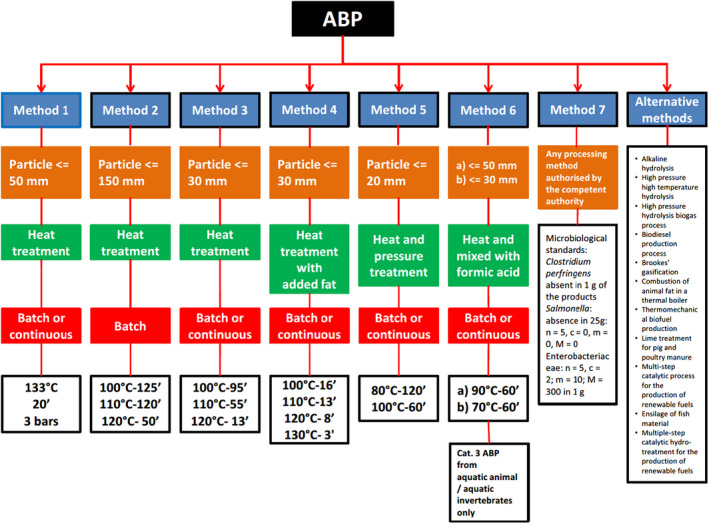
Figure B.1: Processing methods of ABP according to Commission Regulation (EU) No 142/2011
Appendix C – Report on expert knowledge elicitation
1.
1.1.
1.1.1.
Description and methodology
The EKE (expert knowledge elicitation) questions concerned three or four indicators, eight materials as listed in the ToR and 16 combinations, making a total of 52 data points to be assessed.
The EKE question was specified as follows: ‘What is the probability that a X log10 reduction of the Y indicator is achieved in more than 99% of the cases, by application of the process as indicated in the table, to the material(s) as indicated in the table, assuming that the process is performed as prescribed and that the indicated process conditions are achieved?’.
Y could be S. Senftenberg (775W, H2S negative), E. faecalis, the most resistant of the viruses (as per hazard identification or parvovirus if none was identified) or eggs of Ascaris sp.
X is 5 for S. Senftenberg (775W, H2S negative) and E. faecalis, or 3 for the most resistant of the viruses (as per hazard identification or parvovirus if none was identified) or eggs of Ascaris sp.
It is assumed that the standard process is correctly performed, under the conditions indicated by the process parameters, and as described in the opinion. Variability in process performance is not to be considered in this assessment. However, even without any variation in process performance, the log reduction achieved may vary to some extent from case to case. The question to answer is whether the target log10 reduction will be achieved in more than 99% of cases, because 100% may be too unrealistic and would become dependent on ‘exceptional cases’. Thus, the ‘probability’ in the question refers to uncertainty, not variability. Specifically, it expresses the degree of certainty that the target log10 reduction will be achieved in more than 99% of the cases.
The EKE consisted of two steps:
-
–
Step 1: individual judgements (14 July to 16 August 2021)
-
–
Step 2: consensus judgements (20 August 2021)
The experts comprised six Working Group (WG) members developing the opinion (one extra WG member resigned before Step 2 and did not provide individual judgement), plus two EFSA scientists who were supporting the WG. The elicitation was facilitated by an elicitor (hearing expert). A member of the EFSA scientific staff was appointed as rapporteur.
The EKE section was recorded, only as a support to prepare the notes. This recording has been removed to assure anonymity of the experts.
Step 1: Individual judgements
Training was delivered to all participants on the general concept of probability, EFSA's approximate probability scale, uncertainty, variability and EKE.
During Step 1, the participants had nearly 5 weeks to provide individual judgements for each of the 52 combinations by considering them separately, taking into account the version of the draft opinion at the beginning of the process (14 July 2021) with the raw data on thermal and chemical inactivation of the indicators, the description of the processes, the integration of the evidence and the uncertainty table, as well as the personal expertise and assessment of the uncertainties involved. For this purpose, the experts received a spreadsheet with a template to provide their answers. They did not discuss their judgements with other experts at this stage.
The answer for each combination was given as a probability range that reflects the expert's degree of certainty that the indicated log10 reduction is achieved. These probability ranges could be one of those given in the approximate probability scale presented in EFSA's uncertainty guidance or any other. The participants were encouraged to give explanations of the reasons for each subjective probability range.
The options included the template were:
| 99–100% (almost certain) |
| 95–99% (extremely likely) |
| 90–95% (very likely) |
| 66–90% (likely) |
| 33–66% (about as likely as not) |
| 10–33% (unlikely) |
| 5–10% (very unlikely) |
| 1–5% (extremely unlikely) |
| 0–1% (almost impossible) |
| 100% (certain) |
| 50–100% (more likely than not) |
| 0–50% (more unlikely than likely) |
| 0–100% (inconclusive) |
| Other (to be defined by the participant) |
Step 2: Consensus judgements
The next step was to reach a consensus judgement for each of the 52 data points. During the open session in the WG meeting, for each combination of materials and processes (16), three or four probabilities were discussed at a time depending on the number of indicators. It was explained that the consensus is not an average of the individual judgements, or a compromise where some experts defer to the judgement of other participants. The experts were asked to consider what a rational impartial observer (RIO) would judge, having considered the evidence, uncertainties, the individual judgements and having heard the discussion.
After getting an overview of the individual expert judgements obtained in step 1, at the beginning of the EKE session, the participants expressed the rationale behind their individual judgements, to clarify potential generic biases in their judgements.
As proposed by the team preparing the EKE, the elicitor and a WG member, the discussion started for each combination with the standard subjective probability ranges associated with the mean of the median estimates of all the individual ranges as a proposed consensus, avoiding the discussion of individual opinions per se. During the discussion, there was a focus on the evidence as presented in the opinion and consistency in the probability ranges obtained for the different combinations.
The stepwise approach applied for each of the combinations consisted of the following actions:
Recall material/process description and target log10 reduction.
Show summary sheet and proposed consensus.
Display graphs showing expert ranges.
Invite responses to the proposed consensus ranges for the indicators – are they what an RIO would think?
Review individual judgements for selected indicators if helpful.
Discuss proposed adjustments to consensus (if any).
Confirm consensus – if not reached, invite experts to review/revise own judgements and submit them after the meeting.
Check that the notes taken have captured the key reasons for the consensus judgements.
Results of Step 1: Individual judgements
Individual judgements were obtained from eight experts. The results are illustrated in Figures C.1 and C.2.
Figure C.1.
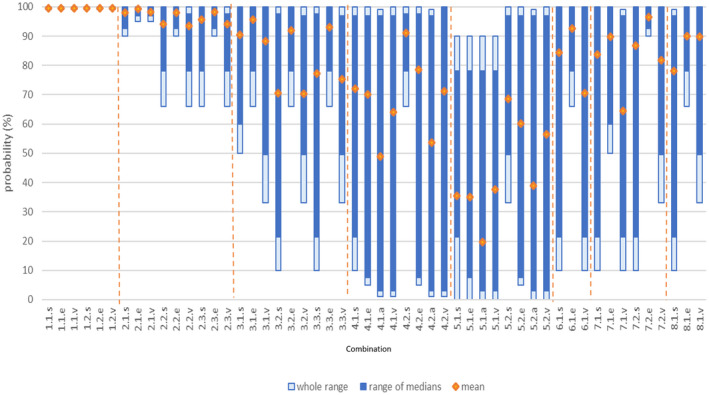
- Combinations are indicated as material number.Process number.Indicator (s: S. senftenberg; e: E. faecalis; a: eggs of Ascaris sp; v: virus). The bars indicate the whole range of elicited probabilities (lowest lower value of the ranges to highest upper value of the ranges) and the range of the medians of the ranges provided by the experts. The dots show the mean of the median estimates provided by the experts.
Figure C.2.

- The red bars indicate the range of the mean of the lowest estimates of the ranges provided by the expert to the mean of the upper estimates of these ranges (M) and the whole range of elicited probabilities (WR).
The figures show that there is large agreement between individual experts for some of the combinations, and large disagreement for others. These results were discussed during Step 2 of the EKE.
Individual expert's rationales for the individual judgements, as discussed at the start of Step 2
In some cases, the evidence was scarce.
Prior assumption was that the probability would be high.
Decisions were supported by calculations using predictive microbiology (temperature and pH) tools despite the differences in the nature of the material.
Judgement based on the extracted data in the opinion trying to narrow probability ranges.
Linearity was not assumed but additive effect for the different treatments and that indicators in the material under investigation would be harder to inactivate than in the evidence.
Broad ranges were considered at first. Easier for thermal treatments while more uncertain for chemical treatments
Judgement started by indicator and used the evidence from the opinion, supported by background knowledge and giving priority to some papers.
Judgement based on evidence in the opinion.
Judgement based on quantitative data in the figures of the opinion for bacteria and tables for viruses. Less confident on chemical processes.
Results of the consensus for each combination
After discussion among the experts, consensus was achieved on the probability ranges that were considered to best represent the uncertainty on whether the indicated log reductions are achieved with the standard processes for the different materials. Consensus implied that the experts agreed that an RIO, considering the evidence and following the discussion, would conclude that the elicited probability range was appropriate. Table C.1 provides the main arguments for obtaining these ranges, for each of the 16 processes.
Annex A – Protocol
1.
Annex A can be found in the online version of this output (‘Supporting information’ section): https://efsa.onlinelibrary.wiley.com/doi/10.2903/j.efsa.2021.6932#efs2(Rev)6932-sup-0001
Supporting information
Protocol
Suggested citation: EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards) , Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L, Hilbert F, Lindqvist R, Nauta M, Peixe L, Ru G, Simmons M, Skandamis P, Suffredini E, Bottari B, Cummins E, Ylivainio K, Muñoz Guajardo I, Ortiz‐Pelaez A and Alvarez‐Ordóñez A, 2021. Inactivation of indicator microorganisms and biological hazards by standard and/or alternative processing methods in Category 2 and 3 animal by‐products and derived products to be used as organic fertilisers and/or soil improvers. EFSA Journal 2021;19(12):6932, 111 pp. 10.2903/j.efsa.2021.6932
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00401
Panel members: Ana Allende, Avelino Alvarez‐Ordóñez, Declan Bolton, Sara Bover‐Cid, Marianne Chemaly, Robert Davies, Alessandra De Cesare, Lieve Herman, Friederike Hilbert, Konstantinos Koutsoumanis, Roland Lindqvist, Maarten Nauta, Luisa Peixe, Giuseppe Ru, Marion Simmons, Panagiotis Skandamis and Elisabetta Suffredini.
Declarations of interest: The declarations of interest of all scientific experts active in EFSA's work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: The Panel wishes to thank Paul Gale, Working Group member until his resignation on 19 August 2021, for his contributions to the discussions. The Panel also wishes to thank Katrin Bote, Maria Francesca Iulietto and Winy Messens for their contributions to the drafting of this scientific output.
Reproduction of the images listed below is prohibited and permission must be sought directly from the copyright holder: Figure 1: © Toldrá‐Reig, F., Mora, L., Toldrá, F.
Adopted: 20 October 2021
Notes
Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by‐products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by‐products Regulation, OJ L 300, 14.11.2009, p. 1).
Regulation (EU) 2017/625 of the European Parliament and of the Council of 15 March 2017 on official controls and other official activities performed to ensure the application of food and feed law, rules on animal health and welfare, plant health and plant protection products, (OJ L 95, 7.4.2017, p. 1).
Regulation (EU) 2019/1009 of the European Parliament and of the Council of 5 June 2019 laying down rules on the making available on the market of EU fertilising products and amending Regulations (EC) No 1069/2009 and (EC) No 1107/2009 and repealing Regulation (EC) No 2003/2003. OJ L 170, 25.6.2019, p. 1.
In the original text of Article 5(2) of Regulation (EC) No 1069/2009, the end point in the manufacturing chain of OF/SI was not included in order to prevent the transmission of transmissible spongiform encephalopathies (TSE/BSE).
As defined in Article 32(1)(a) of Regulation (EC) No 1069/2009.
Regulation (EC) No 999/2001 of the European Parliament and of the Council of 22 May 2001 laying down rules for the prevention, control and eradication of certain transmissible spongiform encephalopathies. OJ L 147, 31.5.2001, p. 1.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive. OJ L 054 26.2.2011, p. 1.
EFSA Journal 2005; 264:1–21.
EFSA Journal 2009; 7(11):1370.
EFSA Journal 2015; 13(11):4306.
EFSA Journal 2005:257:1–30.
Article 6 of and Annex III to Commission Regulation (EU) No 142/2011.
EFSA Journal 2004; 23:1–3.
EFSA Journal 2010; 8(12):1934.
EFSA Journal 2011; 9(2):1976.
EFSA Journal 2015; 13(11):4307.
Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. OJ L 31, 1.2.2002, p. 1.
Commission Regulation (EU) No 142/2011 of 25 February 2011 implementing Regulation (EC) No 1069/2009 of the European Parliament and of the Council laying down health rules as regards animal by‐products and derived products not intended for human consumption and implementing Council Directive 97/78/EC as regards certain samples and items exempt from veterinary checks at the border under that Directive.
Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the incineration of waste OJ L 332, 28.12.2000, pp. 91–111.
International Committee on Taxonomy of Viruses (http://www.ictvonline.com)
Directive 2000/76/EC of the European Parliament and of the Council of 4 December 2000 on the incineration of waste, as amended. https://eur-lex.europa.eu/eli/dir/2000/76/oj
Esterification is not required for processed fat derived from Category 3 material.
References
- Abu‐Orf MM, Brewster J, Oleszkiewicz J, Reimers RS, Lagasse P, Amy B and Glindemann D, 2004. Production of class A biosolids with anoxic low dose alkaline treatment and odor management. Water Science and Technology, 49, 131–138. 10.2166/wst.2004.0626 [DOI] [PubMed] [Google Scholar]
- Abutarbush SM, Hananeh WM, Ramadan W, Al Sheyab OM, Alnajjar AR, Al Zoubi IG, Knowles NJ, Bachanek‐Bankowska K and Tuppurainen ESM, 2016. Adverse reactions to field vaccination against lumpy skin disease in jordan. Transboundary and Emerging Diseases, 63, e213–e219. 10.1111/tbed.12257 [DOI] [PubMed] [Google Scholar]
- Aguirre J, Pin C, Rodriguez M and Garcia de Fernando G, 2009. Analysis of the variability in the number of viable bacteria after mild heat treatment of food. Applied and Environmental Microbiology, 75, 6992–6997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken MD, Sobsey MD, Shehee M, Blauth KE, Hill VR, Farrell JB, Nappier SP, Walters GW, Crunk PL and Van Abel N, 2005. Laboratory evaluation of thermophilic‐anaerobic digestion to produce class A biosolids. 2. Inactivation of pathogens and indicator organisms in a continuous‐flow reactor followed by batch treatment. Water Environment Research, 77, 3028–3036. [DOI] [PubMed] [Google Scholar]
- Álvarez‐Ordóñez A, Fernández A, Bernardo A and López M, 2009. A comparative study of thermal and acid inactivation kinetics in fruit juices of salmonella enterica serovar typhimurium and salmonella enterica serovar senftenberg grown at acidic conditions. Foodborne Pathogens and Disease, 6, 1147–1155. 10.1089/fpd.2009.0313 [DOI] [PubMed] [Google Scholar]
- Aly S and Gaber A, 2007. Inactivation of foot and mouth disease virus in milk and milk products. Milchwissenschaft, 62, 3–5. [Google Scholar]
- Azmi NA, Idris A and Yusof NSM, 2018. Ultrasonic technology for value added products from feather keratin. Ultrasonics Sonochemistry, 47, 99–107. [DOI] [PubMed] [Google Scholar]
- Babiuk S, Bowden TR, Boyle DB, Wallace DB and Kitching RP, 2008. Capripoxviruses: an emerging worldwide threat to sheep, goats and cattle. Transboundary and Emerging Diseases, 55, 263–272. 10.1111/j.1865-1682.2008.01043.x [DOI] [PubMed] [Google Scholar]
- Bachrach HL, 1959. Foot‐and‐mouth disease virus: stability of its ribonucleic acid core to acid and to heat. Biochemical and Biophysical Research Communications, 1, 356–360. 10.1016/0006-291X(59)90055-5 [DOI] [Google Scholar]
- Bachrach HL, Breese SS, Callis JJ, Hess WR and Patty RE, 1957. Inactivation of foot‐and-mouth disease virus by pH and temperature changes and by formaldehyde. 10.3181/00379727-95-23148 [DOI] [PubMed] [Google Scholar]
- Balamurugan V, Venkatesan G, Bhanuprakash V and Singh RK, 2013. Camelpox, an emerging orthopox viral disease. Indian Journal of Virology, 24, 295–305. 10.1007/s13337-013-0145-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bean CL, Hansen JJ, Margolin AB, Balkin H, Batzer G and Widmer G, 2007. Class B alkaline stabilization to achieve pathogen inactivation. International Journal of Environmental Research and Public Health, 4, 53–60. 10.3390/ijerph2007010009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato MS, Capua I and Alexander DJ, 2009. Avian influenza viruses in poultry products: a review. Avian Pathology, 38, 193–200. 10.1080/03079450902912200 [DOI] [PubMed] [Google Scholar]
- Behera BK, Pradhan PK, Swaminathan TR, Sood N, Paria P, Das A, Verma DK, Kumar R, Yadav MK, Dev AK, Parida PK, Das BK, Lal KK and Jena JK, 2018. Emergence of Tilapia Lake Virus associated with mortalities of farmed Nile Tilapia Oreochromis niloticus (Linnaeus 1758) in India. Aquaculture, 484, 168–174. 10.1016/j.aquaculture.2017.11.025 [DOI] [Google Scholar]
- Berechet MD, Niculescu M, Gaidau C, Ignat M and Epure DG, 2018. Alkaline‐enzymatic hydrolyses of wool waste for different applications. Rev Chim‐Bucharest, 69, 1649–1654. [Google Scholar]
- Bhavsar P, Zoccola M, Patrucco A, Montarsolo A, Rovero G and Tonin C, 2017. Comparative study on the effects of superheated water and high temperature alkaline hydrolysis on wool keratin. Textile Research Journal, 87, 1696–1705. [Google Scholar]
- Biosecurity New Zealand (Ministry of Agriculture and Forestry, Wellington), 2007. Import risk analysis: hides and skins from specified animals. Draft for public consultation. Available online: https://www.agriculture.govt.nz/dmsdocument/6142/direct
- Blümel J, Schmidt I, Willkommen H and Löwer J, 2002. Inactivation of parvovirus B19 during pasteurization of human serum albumin. Transfusion, 42, 1011–1018. 10.1046/j.1537-2995.2002.00158.x [DOI] [PubMed] [Google Scholar]
- Bocaneti F, Altamura G, Corteggio A, Velescu E, Roperto F and Borzacchiello G, 2016. Bovine papillomavirus: new insights into an old disease. Transboundary and Emerging Diseases, 63, 14–23. 10.1111/tbed.12222 [DOI] [PubMed] [Google Scholar]
- Borzacchiello G, Russo V, Della Salda L, Roperto S and Roperto F, 2008. Expression of platelet‐derived growth factor‐β receptor and bovine papillomavirus E5 and E7 oncoproteins in equine sarcoid. Journal of Comparative Pathology, 139, 231–237. 10.1016/j.jcpa.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Bosch A, Lucena F and Jofre J, 1986. Fate of human enteric viruses (rotaviruses and enteroviruses) in sewage after primary sedimentation. Water Science and Technology, 18, 47–52. 10.2166/wst.1986.0110 [DOI] [Google Scholar]
- Bräuniger S, Fischer I and Peters J, 1994. The temperature stability of bovine parvovirus. Zentralblatt fur Hygiene und Umweltmedizin= International journal of hygiene and environmental medicine, 196, 270–278. [PubMed] [Google Scholar]
- Bräuniger S, Peters J, Borchers U and Kao M, 2000. Further studies on thermal resistance of bovine parvovirus against moist and dry heat. International Journal of Hygiene and Environmental Health, 203, 71–75. 10.1078/S1438-4639(04)70010-3 [DOI] [PubMed] [Google Scholar]
- Brisolara KF and Reimers RS, 2013. Advances in advanced alkaline stabilization and disinfection. 2715–2722 pp. [Google Scholar]
- Brodersen BW, 2004. Immunohistochemistry used as a screening method for persistent bovine viral diarrhea virus infection. Veterinary Clinics of North America ‐ Food Animal Practice, 20, 85–93. 10.1016/j.cvfa.2003.11.007 [DOI] [PubMed] [Google Scholar]
- Burtscher C and Wuertz S, 2003. Evaluation of the use of PCR and reverse transcriptase PCR for detection of pathogenic bacteria in biosolids from anaerobic digestors and aerobic composters. Applied and Environmental Microbiology, 69, 4618–4627. 10.1128/AEM.69.8.4618-4627.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwirhonde F, Bulambo G, Mutelezi F, Kasai F and Kadima J, 2018. Evaluation of protein and mineral nutrients in cattle hide scraps used for treating children with kwashiorkor in bukavu. Journal of Nutritional Health and Food Engineering, 8. 10.15406/jnhfe.2018.08.00256 [DOI] [Google Scholar]
- Callan RJ, Schnackel JA, Van Campen H, Mortimer RG, Cavender JA and Williams ES, 2002. Percutaneous collection of fetal fluids for detection of bovine viral diarrhea virus infection in cattle. Journal of the American Veterinary Medical Association, 220, 1348–1352. 10.2460/javma.2002.220.1348 [DOI] [PubMed] [Google Scholar]
- Callegaro K, Brandelli A and Daroit DJ, 2019. Beyond plucking: feathers bioprocessing into valuable protein hydrolysates. Waste Management, 95, 399–415. 10.1016/j.wasman.2019.06.040 [DOI] [PubMed] [Google Scholar]
- Capizzi‐Banas S, Deloge M, Remy M and Schwartzbrod J, 2004. Liming as an advanced treatment for sludge sanitisation: helminth eggs elimination ‐ Ascaris eggs as model. Water Research, 38, 3251–3258. 10.1016/j.watres.2004.04.015 [DOI] [PubMed] [Google Scholar]
- Carn VM, 1993. Control of capripoxvirus infections. Vaccine, 11, 1275–1279. 10.1016/0264-410X(93)90094-E [DOI] [PubMed] [Google Scholar]
- Ceylan E and Bautista DA, 2015. Evaluating Pediococcus acidilactici and Enterococcus faecium NRRL B‐2354 as thermal surrogate microorganisms for Salmonella for in‐plant validation studies of low‐moisture pet food products. Journal of Food Protection, 78, 934–939. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay B, Goswami AR, Aich A, Datta S and Mukhopadhyay S, 2011. Characterization of tannery solid wastes based fertilizers and fish‐food. The Journal of Solid Waste Technology and Management, 37, 253–259. 10.5276/jswtm.2011.253 [DOI] [Google Scholar]
- Ciavatta C, Manoli C, Cavani L, Franceschi C and Sequi P, 2012. Chromium‐containing organic fertilizers from tanned hides and skins: a review on chemical, environmental, agronomical and legislative aspects. Journal of Environmental Protection, 03, 1532–1541. 10.4236/jep.2012.311169 [DOI] [Google Scholar]
- Corte L, Dell'abate MT, Magini A, Migliore M, Felici B, Roscini L, Sardella R, Tancini B, Emiliani C, Cardinali G and Benedetti A, 2014. Assessment of safety and efficiency of nitrogen organic fertilizers from animal‐based protein hydrolysates–a laboratory multidisciplinary approach. Journal of the Science of Food and Agriculture, 94, 235–245. 10.1002/jsfa.6239 [DOI] [PubMed] [Google Scholar]
- Cotmore SF, Agbandje‐McKenna M, Canuti M, Chiorini JA, Eis‐Hubinger A‐M, Hughes J, Mietzsch M, Modha S, Ogliastro M, Pénzes JJ, Pintel DJ, Qiu J, Soderlund‐Venermo M, Tattersall P, Tijssen P and Consortium IR, 2019. ICTV virus taxonomy profile: parvoviridae. Journal of General Virology, 100, 367–368. 10.1099/jgv.0.001212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaudier M and Denesvre C, 2014. Marek's disease virus and skin interactions. Veterinary Research, 45. 10.1186/1297-9716-45-36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couteaudier M, Courvoisier K, Trapp‐Fragnet L, Denesvre C and Vautherot JF, 2016. Keratinocytes derived from chicken embryonic stem cells support Marek's disease virus infection: a highly differentiated cell model to study viral replication and morphogenesis. Virology Journal, 13. 10.1186/s12985-015-0458-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CPRAC (Regional Activity Centre for Sustainable Consumption and Production), Online. Example ‐ leather industry: pellneta. Available online: http://www.cprac.org/en/static/DAOM/ing/html/exem2.htm
- Damaso CRA, Esposito JJ, Condit RC and Moussatché N, 2000. An emergent poxvirus from humans and cattle in Rio de Janeiro state: cantagalo virus may derive from brazilian smallpox vaccine. Virology, 277, 439–449. 10.1006/viro.2000.0603 [DOI] [PubMed] [Google Scholar]
- Davidson I and Skoda I, 2005. The impact of feathers use on the detection and study of DNA viral pathogens in commercial poultry. World's Poultry Science Journal, 61, 407–417+516+521+525. 10.1079/WPS200452 [DOI] [Google Scholar]
- Davidson PM and Taylor TM, 2001. Chemical preservatives and natural antimicrobial compounds. In: Doyle MP and Lrba TJM (eds.). Food Microbiology: Fundamentals and Frontiers. ASM Press, Washington, DC. [Google Scholar]
- Davies FG, 1991. Lumpy skin disease, an African capripox virus disease of cattle. British Veterinary Journal, 147, 489–503. 10.1016/0007-1935(91)90019-J [DOI] [PubMed] [Google Scholar]
- Denesvre C, 2013. Marek's disease virus morphogenesis. Avian Diseases, 57, 340–350. 10.1637/10375-091612-Review.1 [DOI] [PubMed] [Google Scholar]
- Donaldson NS, Kosmider R, Reed N and Gale P (Defra), 2011. Assessment of the thermo‐stability of selected viruses that pose a relevant hazard in Category 3 animal by‐products used as incoming materials in biogas and composting plants.
- Doyle ME and Mazzotta AS, 2000. Review of studies on the thermal resistance of Salmonellae. Journal of Food Protection, 63, 779–795. 10.4315/0362-028x-63.6.779 [DOI] [PubMed] [Google Scholar]
- Dudley JP, 2008. Public health and epidemiological considerations for avian influenza risk mapping and risk assessment. Ecology and Society, 13. 10.5751/ES-02548-130221 [DOI] [Google Scholar]
- EFSA AHAW Panel and EFSA BIOHAZ Panel (EFSA Panels on Animal Health and Welfare and on Biological Hazards), 2011. Scientific Opinion on Hatchery Waste as animal by‐products. EFSA Journal 2011;9(7):2321, 77 pp. https://efsa.onlinelibrary.wiley.com/doi/pdfdirect/2310.2903/j.efsa.2011.2321 [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Scientific Panel on Biological hazards) , 2010. Scientific Opinion on Lime Treatment of Solid Pig and Poultry Manure. EFSA Journal 2010;8(7):1681, 23 pp. 10.2903/j.efsa.2010.1681 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Scientific Panel on Biological hazards), 2011. Scientific Opinion on the capacity of oleochemical processes to minimise possible risks linked to TSE in Category 1 animal by‐products. EFSA Journal 2011;9(2):1976, 26 pp. https://efsa.onlinelibrary.wiley.com/doi/epdf/1910.2903/j.efsa.2011.1976 [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), 2015. Scientific Opinion on a continuous multiple‐step catalytic hydro‐treatment for the processing of rendered animal fat (Category 1). EFSA Journal 2015;13:4307, 34 pp. 10.2903/j.efsa.2015.4307 [DOI] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Ricci A, Allende A, Bolton D, Chemaly M, Davies R, Girones R, Herman L, Koutsoumanis K and Lindqvist R, 2017. Evaluation of the Application for new alternative biodiesel production process for rendered fat of Cat 1 (BDI‐RepCat process, AT). EFSA Journal 2017;15(11):5053, 22 pp. 10.2903/j.efsa.2017.5053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L and Hilbert F, 2020. Evaluation of an alternative method for production of biodiesel from processed fats derived from Category 1, 2 and 3 animal by‐products (submitted by College Proteins). EFSA Journal 2020;18:6089, 44 pp. 10.2903/j.efsa.2020.6089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA BIOHAZ Panel (EFSA Panel on Biological Hazards), Koutsoumanis K, Allende A, Bolton D, Bover‐Cid S, Chemaly M, Davies R, De Cesare A, Herman L and Hilbert F, 2021. Evaluation of the application for new alternative biodiesel production process for rendered fat including Category 1 animal by‐products (BDI‐RepCat® process, AT). EFSA Journal 2021;19(4):6511, 44 pp. 10.2903/j.efsa.2021.6511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018a. Guidance on Uncertainty Analysis in Scientific Assessments. EFSA Journal 2018;16(1):5123, 39 pp. 10.2903/j.efsa.2018.5123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA Scientific Committee , 2018b. Scientific Opinion on the principles and methods behind EFSA's Guidance on Uncertainty Analysis in Scientific Assessment. EFSA Journal 2018;16(1):5122, 235 pp. 10.2903/j.efsa.2018.5122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Senousy WM, Shalaby M, Deeb AMM and Alhawary II, 2020. Thermal inactivation of hepatitis a virus, noroviruses, and simian rotavirus in cows’ milk. Food and Environmental Virology, 12, 310–320. 10.1007/s12560-020-09443-z [DOI] [PubMed] [Google Scholar]
- Elving J, Vinnerås B, Albihn A and Ottoson JR, 2014. Thermal treatment for pathogen inactivation as a risk mitigation strategy for safe recycling of organic waste in agriculture. Journal of Environmental Science and Health – Part B Pesticides, Food Contaminants, and Agricultural Wastes, 49, 679–689. 10.1080/03601234.2014.922783 [DOI] [PubMed] [Google Scholar]
- Endale YT, Yirsaw BD and Asfaw SL, 2012. Pathogen reduction efficiency of on‐site treatment processes in eco‐sanitation system. Waste Management and Research, 30, 750–754. 10.1177/0734242X11432190 [DOI] [PubMed] [Google Scholar]
- ENEA (Italian National Agency for New Technologies EaSED), 2020. Manual for the employ of deodorised poultry manure in the bating phase. Available online: https://pdc.minambiente.it/sites/default/files/progetti/podeba_manual.pdf
- Eriksen L, Andreasen P and Ilsøe B, 1996. Inactivation of Ascaris suum eggs during storage in lime treated sewage sludge. Water Research, 30, 1026–1029. 10.1016/0043-1354(95)00258-8 [DOI] [Google Scholar]
- Espersen R, Falco FC, Hagglund P, Gernaey KV, Lantz AE and Svensson B, 2020. Two novel S1 peptidases from Amycolatopsis keratinophila subsp. keratinophila D2(T) degrading keratinous slaughterhouse by‐products. Applied Microbiology and Biotechnology, 104, 2513–2522. 10.1007/s00253-020-10380-x [DOI] [PubMed] [Google Scholar]
- European Commission , online. The leather industry in the EU. Available online: https://ec.europa.eu/growth/sectors/fashion/leather/eu-industry_fr [Accessed: 3 November 2020].
- Falco FC, Espersen R, Svensson B, Gernaey KV and Eliasson Lantz A, 2019. An integrated strategy for the effective production of bristle protein hydrolysate by the keratinolytic filamentous bacterium Amycolatopsis keratiniphila D2. Waste Management, 89, 94–102. 10.1016/j.wasman.2019.03.067 [DOI] [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization), 1996. Management of waste from animal product processing. 3. Tanneries. Available online: http://www.fao.org/3/X6114E/x6114e05.htm
- FAO (Food and Agriculture Organization), 2012. Biofuel co‐products as livestock feed – opportunities and challenges. Makkar HPS (ed.). Rome. Available online: http://www.fao.org/3/i3009e/i3009e.pdf
- Fatoba AJ and Adeleke MA, 2019. Chicken anemia virus: a deadly pathogen of poultry. Acta Virologica, 63, 19–25. 10.4149/av_2019_110 [DOI] [PubMed] [Google Scholar]
- Felizardo P, Correia MJ, Raposo I, Mendes JF, Berkemeier R and Bordado JM, 2006. Production of biodiesel from waste frying oils. Waste Management, 26, 487–494. 10.1016/j.wasman.2005.02.025 [DOI] [PubMed] [Google Scholar]
- Ferreira BC, Ecco R, Couto RM, Coelho HE, Rossi DA, Beletti ME and Silva PL, 2018. Outbreak of cutaneous form of avian poxvirus disease in previously pox‐vaccinated commercial turkeys. Pesquisa Veterinaria Brasileira, 38, 417–424. 10.1590/1678-5150-PVB-4463 [DOI] [Google Scholar]
- Fitzmorris KB, Reimers RS, Oleszkiewicz JA and Little MD, 2007. Decrease of time for pathogen inactivation in alkaline disinfection systems using pressure. Water Environment Research, 79, 388–395. 10.2175/106143006X111862 [DOI] [PubMed] [Google Scholar]
- Flach B, Lieberz S and Bolla S, online. EU Biofuels Annual 200, Gain Report E42020‐0032, USDA Foreign Agricultural Service. Available online: https://apps.fas.usda.gov/newgainapi/api/Report/
- Flagstad A, 1972. Isolation and classification of feline picornavirus and herpesvirus in Denmark. Acta Veterinaria Scandinavica, 13, 462–471. 10.1186/BF03547152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SB, Wise LM and Mercer AA, 2015. Molecular genetic analysis of orf virus: poxvirus that has adapted to skin. Viruses, 7, 1505–1539. 10.3390/v7031505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming SB, McCaughan C, Lateef Z, Dunn A, Wise LM, Real NC and Mercer AA, 2017. Deletion of the chemokine binding protein gene from the parapoxvirus ORF virus reduces virulence and pathogenesis in sheep. Frontiers in Microbiology, 8, 46. 10.3389/fmicb.2017.00046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbraith H, 2000. Protein and sulphur amino acid nutrition of hair fibre‐producing Angora and Cashmere goats. Livestock Production Science, 64, 81–93. [Google Scholar]
- Galbraith H, 2010a. Animal fibre: connecting science and production. Animal, 4, 1447–1450. [Google Scholar]
- Galbraith H, 2010b. Fundamental hair follicle biology and fine fibre production in animals. Animal, 4, 1490–1509. [DOI] [PubMed] [Google Scholar]
- Galbraith H, 2019. Animal fibre production in Europe: biology, species, breeds and contemporary utilisation. In: Gerken M, Renieri C, Allain D, Galbraith H, Gutiérrez JP, McKenna L, Niznikowski R and Wurzinger M (eds.). Advances in Fibre Production Science in South American Camelids and Other Fibre Animals. Universitätsverlag Göttingen, Göttingen, Germany. pp. 23–41. [Google Scholar]
- Gale P, Kelly L and Snary EL, 2016. Qualitative assessment of the entry of capripoxviruses into Great Britain from the European Union through importation of ruminant hides, skins and wool. Microbial Risk Analysis, 1, 13–18. 10.1016/j.mran.2015.07.001 [DOI] [Google Scholar]
- Gebremariam SN and Marchetti JM, 2018. Economics of biodiesel production. Energy Conversion and Management, 168, 74–84. 10.1016/j.enconman.2018.05.002 [DOI] [Google Scholar]
- Gelaye E and Lamien CE, 2019. Lumpy Skin Disease and Vectors of LSDV. Springer International Publishing. pp. 267–288. [Google Scholar]
- Ghiglietti R, Genchi C, Di Matteo L, Calcaterra E and Colombi A, 1997. Survival of Ascaris suum eggs in ammonia‐treated wastewater sludges. Bioresource Technology, 59, 195–198. 10.1016/S0960-8524(96)00147-2 [DOI] [Google Scholar]
- Goddard MR, Bates J and Butler M, 1982. Isolation of indigenous enteroviruses from chemically treated and dewatered sludge samples. Applied and Environmental Microbiology, 44, 1042–1046. 10.1128/aem.44.5.1042-1046.1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goerner‐Hu X, Scott EL, Seeger T, Schneider O and Bitter JH, 2020. Reaction stages of feather hydrolysis: factors that influence availability for enzymatic hydrolysis and cystine conservation during thermal pressure hydrolysis. Biotechnology and Bioprocess Engineering, 25, 749–757. 10.1007/s12257-019-0351-8 [DOI] [Google Scholar]
- Gong Q‐L, Wang Q, Yang X‐Y, Li D‐L, Zhao B, Ge G‐Y, Zong Y, Li J‐M, Leng X, Shi K, Liu F and Du R, 2021. Seroprevalence and risk factors of the bluetongue virus in cattle in china from 1988 to 2019: a comprehensive literature review and meta‐analysis. Frontiers in Veterinary Science, 7, 1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalo M, Jespersen CM, Jensen K, Støier S and Meinert L, 2016. Pig bristles – an underestimated biomass resource. Proceedings of the 62nd International Congress of Meat Science and Technology, Proc. Bangkok, Thailand. [Google Scholar]
- Gousterova A, Nustorova M, Goshev I, Christov P, Braikova D, Tishinov K, Haertle T and Nedkov P, 2003. Alkaline hydrolysate of waste sheep wool aimed as fertilizer. Biotechnology and Biotechnological Equipment, 17, 140–145. [Google Scholar]
- Grooms DL and Keilen ED, 2002. Screening of neonatal calves for persistent infection with bovine viral diarrhea virus by immunohistochemistry on skin biopsy samples. Clinical and Diagnostic Laboratory Immunology, 9, 898–900. 10.1128/CDLI.9.4.898-900.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubman MJ and Baxt B, 2004. Foot‐and‐mouth disease. Clinical Microbiology Reviews, 17, 465–493. 10.1128/CMR.17.2.465-493.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbins S, Forster J, Clive S, Schley D, Zuber S, Schaaff J and Corley D, 2016. Thermal inactivation of foot and mouth disease virus in extruded pet food. Revue Scientifique et Technique de l'OIE, 35, 965–972. 10.20506/rst.35.3.2582 [DOI] [PubMed] [Google Scholar]
- Gupta R and Ramnani P, 2006. Microbial keratinases and their prospective applications: an overview. Applied Microbiology and Biotechnology, 70, 21–33. 10.1007/s00253-005-0239-8 [DOI] [PubMed] [Google Scholar]
- Hafez HM, 2005. Governmental regulations and concept behind eradication and control of some important poultry diseases. World's Poultry Science Journal, 61, 569–581+713+717–718+721+724–725+728–729. 10.1079/WPS200571 [DOI] [Google Scholar]
- Haig DM and McInnes CJ, 2002. Immunity and counter‐immunity during infection with the parapoxvirus orf virus. Virus Research, 88, 3–16. 10.1016/S0168-1702(02)00117-X [DOI] [PubMed] [Google Scholar]
- Haig DM and Mercer AA, 1998. Orf. Veterinary Research, 29, 311–326. [PubMed] [Google Scholar]
- Han R, Cladel NM, Reed CA and Christensen ND, 1998. Characterization of transformation function of cottontail rabbit papillomavirus E5 and E8 genes. Virology, 251, 253–263. 10.1006/viro.1998.9416 [DOI] [PubMed] [Google Scholar]
- Harris LJ, Uesugi AR, Abd SJ and McCarthy KL, 2012. Survival of Salmonella Enteritidis PT 30 on inoculated almond kernels in hot water treatments. Food Research International, 45, 1093–1098. 10.1016/j.foodres.2011.03.048 [DOI] [Google Scholar]
- Hayrapetyan H, Nierop Groot MN and Zwietering MH, 2019. Literature search of heat and acid inactivation parameters of viruses relevant for food waste treatment for recycling to pig feed. Refresh, 34 pp. Available online: https://eu-refresh.org/sites/default/files/REFRESH%20supplementary%20report%20D6.7%20Heat%20and%20acid%20inactivation%20parameters.pdf [Google Scholar]
- Hernandez‐Divers SM, Villegas P, Prieto F, Unda JC, Stedman N, Ritchie B, Carroll R and Hernandez‐Divers SJ, 2006. A survey of selected avian pathogens of backyard poultry in northwestern Ecuador. Journal of Avian Medicine and Surgery, 20, 147–158. 10.1647/2005-015R.1 [DOI] [Google Scholar]
- Hess L and Tater K, 2012. Chapter 18 ‐ dermatologic diseases. In: Quesenberry KE and Carpenter JW (eds.). Ferrets, Rabbits, and Rodents, 3rd Edition. St. Louis, WB Saunders. pp. 232–244. [Google Scholar]
- Hodde J and Hiles M, 2002. Virus safety of a porcine‐derived medical device: evaluation of a viral inactivation method. Biotechnology and Bioengineering, 79, 211–216. 10.1002/bit.10281 [DOI] [PubMed] [Google Scholar]
- Huchzermeyer FW, 1997. Animal health risks associated with ostrich products. Revue Scientifique et Technique (International Office of Epizootics), 16, 111–116. 10.20506/rst.16.1.999 [DOI] [PubMed] [Google Scholar]
- Huerter CJ, Alvarez L and Stinson R, 1991. Orf: case report and literature review. Cleveland Clinic Journal of Medicine, 58, 531–534. 10.3949/ccjm.58.6.531 [DOI] [PubMed] [Google Scholar]
- Jamal SM and Belsham GJ, 2013. Foot‐and‐mouth disease: past, present and future. Veterinary Research, 44, 1–14. 10.1016/j.foodres.2011.03.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosinski KW, Margulis NG, Kamil JP, Spatz SJ, Nair VK and Osterrieder N, 2007. Horizontal transmission of Marek's disease virus requires US2, the UL13 protein kinase, and gC. Journal of Virology, 81, 10575–10587. 10.1128/JVI.01065-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen PK, Phuc PD, Konradsen F, Klank LT and Dalsgaard A, 2009. Survival of Ascaris eggs and hygienic quality of human excreta in Vietnamese composting latrines. Environmental Health: A Global Access Science Source, 8, 57. 10.1186/1476-069X-8-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez B, Barrios JA and Maya C, 2000. Class b biosolids production from wastewater sludge with high pathogenic content generated in an advanced primary treatment. Water Science and Technology, 42, 103–110. 10.2166/wst.2000.0181 [DOI] [Google Scholar]
- Joardar JC and Rahman MM, 2018. Poultry feather waste management and effects on plant growth. International Journal of Recycling of Organic Waste in Agriculture, 7, 183–188. 10.1007/s40093-018-0204-z [DOI] [Google Scholar]
- JRC (Joint Research Institute), 2013. Best available techniques (BAT) reference document for the tanning of hides and skins. JRC reference reports, European Commission, Joint Research Centre, Institute for Prospective Technological Studies. [Google Scholar]
- Kaden V, Lange E, Faust A and Teifke JP, 2007. Value of skin punch biopsies for the diagnosis of acute classical swine fever. Journal of Veterinary Diagnostic Investigation, 19, 697–701. 10.1177/104063870701900614 [DOI] [PubMed] [Google Scholar]
- Kalaiyarasu S, Mishra N, Rajukumar K, Behera SP, Jhade SK and Singh VP, 2019. Development and evaluation of real‐time RT‐PCR using ear hair for specific detection of sheep persistently infected with border disease virus (BDV). Journal of Virological Methods, 269, 55–63. 10.1016/j.jviromet.2019.04.003 [DOI] [PubMed] [Google Scholar]
- Kaleta EF and Hönicke A, 2004. Review of the literature on avian influenza A viruses in pigeons and experimental studies on the susceptibility of domestic pigeons to influenza A viruses of the haemagglutinin subtype H7. Deutsche Tierarztliche Wochenschrift, 111, 467–472. [PubMed] [Google Scholar]
- Kamolsiripichaiporn S, Subharat S, Udon R, Thongtha P and Nuanualsuwan S, 2007. Thermal inactivation of foot‐and‐mouth disease viruses in suspension. Applied and Environmental Microbiology, 73, 7177–7184. 10.1128/AEM.00629-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato S, Fogarty E and Bowman D, 2003. Effect of aerobic and anaerobic digestion on the viability of Cryptosporidium parvum oocysts and Ascaris suum eggs. International Journal of Environmental Health Research, 13, 169–179. 10.1080/0960312031000098071 [DOI] [PubMed] [Google Scholar]
- Kingsley DH, Li X and Chen H, 2014. Temperature effects for high‐pressure processing of picornaviruses. Food and Environmental Virology, 6, 58–61. 10.1007/s12560-013-9131-3 [DOI] [PubMed] [Google Scholar]
- Knight AI, Haines J and Zuber S, 2013. Thermal inactivation of animal virus pathogens. Current Topics in Virology, 11, 103–119. [Google Scholar]
- Koch K and Strauch D, 1981. Removal of polio‐ and parvovirus in sewage‐sludge by lime‐treatment. [Zentralblatt fur Bakteriologie Mikrobiologie und Hygiene – Abt. 1 Orig. B.] Hygiene, 174, 335–347. [PubMed] [Google Scholar]
- Kopit LM, Kim EB, Siezen RJ, Harris LJ and Marco ML, 2014. Safety of the surrogate microorganism Enterococcus faecium NRRL B‐2354 for use in thermal process validation. Applied and Environmental Microbiology, 80, 1899–1909. 10.1128/AEM.03859-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korniłłowicz‐Kowalska T and Bohacz J, 2011. Biodegradation of keratin waste: theory and practical aspects. Waste Management, 31, 1689–1701. 10.1016/j.wasman.2011.03.024 [DOI] [PubMed] [Google Scholar]
- Laba W and Rodziewicz A, 2014. Biodegradation of hard keratins by two bacillus strains. Jundishapur Journal of Microbiology, 7. 10.5812/jjm.8896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Łaba W, Chorążyk D, Pudło A, Trojan‐Piegza J, Piegza M, Kancelista A, Kurzawa A, Żuk I and Kopeć W, 2016. Enzymatic degradation of pretreated pig bristles with crude keratinase of Bacillus cereus PCM 2849. Waste and Biomass Valorization, 8, 527–537. 10.1007/s12649-016-9603-4 [DOI] [Google Scholar]
- Lateef Z, Baird MA, Wise LM, Young S, Mercer AA and Fleming SB, 2010. The chemokine‐binding protein encoded by the poxvirus orf virus inhibits recruitment of dendritic cells to sites of skin inflammation and migration to peripheral lymph nodes. Cellular Microbiology, 12, 665–676. 10.1111/j.1462-5822.2009.01425.x [DOI] [PubMed] [Google Scholar]
- Lee K‐T, Foglia TA and Chang K‐S, 2002. Production of alkyl ester as biodiesel from fractionated lard and restaurant grease. Journal of the American Oil Chemists’ Society, 79, 191–195. 10.1007/s11746-002-0457-y [DOI] [Google Scholar]
- Lelie PN, Reesink HW and Lucas CJ, 1987. Inactivation of 12 viruses by heating steps applied during manufacture of a hepatitis B vaccine. Journal of Medical Virology, 23, 297–301. 10.1002/jmv.1890230313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Woods L, Gerstenberg G, Deng X and Delwart E, 2016. A common parvovirus in deer from California, USA. Journal of Wildlife Diseases, 52, 962–964. 10.7589/2015-12-337 [DOI] [PubMed] [Google Scholar]
- Li K, Wang C, Yang F, Cao W, Zhu Z and Zheng H, 2021. Virus–host interactions in foot‐and‐mouth disease virus infection. Frontiers in Immunology, 12, 206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F and Kitching RP, 2000. Swine vesicular disease: an overview. Veterinary Journal, 160, 192–201. 10.1053/tvjl.2000.0505 [DOI] [PubMed] [Google Scholar]
- Liu Q, Zhang T, Song N, Li Q, Wang Z, Zhang X, Lu X, Fang J and Chen J, 2014. Purification and characterization of four key enzymes from a feather‐degrading Bacillus subtilis from the gut of tarantula Chilobrachys guangxiensis . International Biodeterioration and Biodegradation, 96, 26–32. 10.1016/j.ibiod.2014.08.008 [DOI] [Google Scholar]
- Liu H, Li LX, Sun WC, Shi N, Sun XT, Jin NY and Si XK, 2020. Molecular survey of duck circovirus infection in poultry in southern and southwestern China during 2018 and 2019. BMC Veterinary Research, 16, 80. 10.1186/s12917-020-02301-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund B, Jensen VF, Have P and Ahring B, 1996. Inactivation of virus during anaerobic digestion of manure in laboratory scale biogas reactors. Antonie van Leeuwenhoek, International Journal of General and Molecular Microbiology, 69, 25–31. 10.1007/BF00641608 [DOI] [PubMed] [Google Scholar]
- Lyons B, 2009. Australian Merino wool. In: Proceedings of the Symposium on Natural Fibres. Common Fund for Commodities, Technical Paper no. 56. FAO, Rome, Italy, pp. 83–109. [Google Scholar]
- Ma F and Hanna MA, 1999. Biodiesel production: a review. Bioresource Technology, 70, 1–15. 10.1016/s0960-8524(99)00025-5 [DOI] [Google Scholar]
- Maclachlan NJ, Drew CP, Darpel KE and Worwa G, 2009. The Pathology and pathogenesis of bluetongue. Journal of Comparative Pathology, 141, 1–16. 10.1016/j.jcpa.2009.04.003 [DOI] [PubMed] [Google Scholar]
- Maya C, Ortiz M and Jiménez B, 2010. Viability of Ascaris and other helminth genera non larval eggs in different conditions of temperature, lime (pH) and humidity. Water Science and Technology, 62, 2616–2624. 10.2166/wst.2010.535 [DOI] [PubMed] [Google Scholar]
- McColl K, Westbury H, Kitching R and Lewis V, 1995. The persistence of foot‐and‐mouth disease virus on wool. Australian Veterinary Journal, 72, 286–292. 10.1111/j.1751-0813.1995.tb03556.x [DOI] [PubMed] [Google Scholar]
- McDonnell GE, 2020. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance. ASM Press, John Wiley & Sons. 432 pp. [Google Scholar]
- Meredith AL, 2013. Viral skin diseases of the rabbit. Veterinary Clinics of North America – Exotic Animal Practice, 16, 705–714. 10.1016/j.cvex.2013.05.010 [DOI] [PubMed] [Google Scholar]
- Mijaylova Nacheva P, Moeller G, Chávez X, Ramírez Camperos E and Cardaso Vigueros L, 2002. Characterization and dewaterability of raw and stabilized sludge using different treatment methods. Water Science and Technology, 46, 123–130. 10.2166/wst.2002.0307 [DOI] [PubMed] [Google Scholar]
- Mocé‐Llivina L, Muniesa M, Pimenta‐Vale H, Lucena F and Jofre J, 2003. Survival of bacterial indicator species and bacteriophages after thermal treatment of sludge and sewage. Applied and Environmental Microbiology, 69, 1452–1456. 10.1128/AEM.69.3.1452-1456.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monpoeho S, Maul A, Bonnin C, Patria L, Ranarijaona S, Billaudel S and Ferré V, 2004. Clearance of human‐pathogenic viruses from sludge: study of four stabilization processes by real‐time reverse transcription‐PCR and cell culture. Applied and Environmental Microbiology, 70, 5434–5440. 10.1128/AEM.70.9.5434-5440.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NABC (National Agricultural Biosecurity Center), 2004. Carcass disposal: a comprehensive review. Available online: https://core.ac.uk/download/pdf/5164738.pdf
- Nakajo K, Iwami Y, Komori R, Ishikawa S, Ueno T, Suzuki Y and Takahashi N, 2005. The resistance to acidic and alkaline environments of endodontic pathogen Enterococcus faecalis . International Congress Series, 1284, 191–192. 10.1016/j.ics.2005.06.060 [DOI] [PubMed] [Google Scholar]
- Nandi S, De UK and Chowdhury S, 2011. Current status of contagious ecthyma or orf disease in goat and sheep—a global perspective. Small Ruminant Research, 96, 73–82. 10.1016/j.smallrumres.2010.11.018 [DOI] [Google Scholar]
- Nemeth NM, Young GR, Burkhalter KL, Brault AC, Reisen WK and Komar N, 2009. West Nile virus detection in nonvascular feathers from avian carcasses. Journal of Veterinary Diagnostic Investigation, 21, 616–622. 10.1177/104063870902100505 [DOI] [PubMed] [Google Scholar]
- Nfon CK, Ferman GS, Toka FN, Gregg DA and Golde WT, 2008. Interferon‐α production by swine dendritic cells is inhibited during acute infection with foot‐and‐mouth disease virus. Viral Immunology, 21, 68–77. 10.1089/vim.2007.0097 [DOI] [PubMed] [Google Scholar]
- Ng H, Bayne HG and Garibaldi JA, 1969. Heat resistance of Salmonella: the uniqueness of Salmonella Senftenberg 775W. Applied Microbiology, 17, 78–82. 10.1128/am.17.1.78-82.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedbalski W and Fitzner A, 2019. Senecavirus A: an emerging pathogen causing vesicular disease in pigs. Medycyna Weterynaryjna, 75, 6200. 10.21521/mw.6200 [DOI] [Google Scholar]
- Nims R and Plavsic M, 2013a. Inactivation of caliciviruses. Pharmaceuticals, 6, 358–392. 10.3390/ph6030358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nims R and Plavsic M, 2013b. A proposed modeling approach for comparing the heat inactivation susceptibility of viruses. BioProcessing Journal, 12, 25–35. 10.12665/J122.Nims [DOI] [Google Scholar]
- Nims RW and Plavsic M, 2013c. Intra‐family and inter‐family comparisons for viral susceptibility to heat inactivation. Journal of Microbial and Biochemical Technology, 5, 136–141. 10.4172/1948-5948.1000112 [DOI] [Google Scholar]
- Nims RW and Plavsic M, 2013d. Polyomavirus inactivation – a review. Biologicals, 41, 63–70. 10.1016/j.biologicals.2012.09.011 [DOI] [PubMed] [Google Scholar]
- Nims RW and Zhou SS, 2016. Intra‐family differences in efficacy of inactivation of small, non‐enveloped viruses. Biologicals, 44, 456–462. 10.1016/j.biologicals.2016.05.005 [DOI] [PubMed] [Google Scholar]
- Nuanualsuwan S and Cliver DO, 2003. Capsid functions of inactivated human picornaviruses and feline calicivirus. Applied and Environmental Microbiology, 69, 350–357. 10.1128/AEM.69.1.350-357.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nustorova M, Braikova D, Gousterova A, Vasileva‐Tonkova E and Nedkov P, 2006. Chemical, microbiological and plant analysis of soil fertilized with alkaline hydrolysate of sheep's wool waste. World Journal of Microbiology and Biotechnology, 22, 383–390. [Google Scholar]
- Nyberg KA, Vinnerås B, Lewerin SS, Kjellberg E and Albihn A, 2011. Treatment with Ca(OH)2 for inactivation of Salmonella Typhimurium and Enterococcus faecalis in soil contaminated with infected horse manure. Journal of Applied Microbiology, 110, 1515–1523. 10.1111/j.1365-2672.2011.05006.x [DOI] [PubMed] [Google Scholar]
- Omole D and Ogbiye S, 2013. An evaluation of slaughterhouse wastes in south‐west Nigeria. American Journal of Environmental Protection, 2, 85–89. [Google Scholar]
- Onifade A, Al‐Sane N, Al‐Musallam A and Al‐Zarban S, 1998. A review: potentials for biotechnological applications of keratin‐degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresource Technology, 66, 1–11. 10.1016/S0960-8524(98)00033-9 [DOI] [Google Scholar]
- Ostertag S, 1987. Mikrobiologische – hygienische Untersuchungen über die Anwendung von Branntund Löschkalk zur Klärschlammentseuchung. Dissertation, Vedewa Schriftenreihe, Band 4. Agrar. wiss. Diss., Universität Hohenheim.
- Otterbein CK, Meyer H, Renner‐Müller IC and Munz E, 1996. In vivo and in vitro characterization of two camelpoxvirus isolates with decreased virulence. Revue d’Élevage et de Médecine Vétérinaire des Pays Tropicaux, 49, 114–120. [PubMed] [Google Scholar]
- Ozgunay H, Colak S, Mutlu MM and Akyuz F, 2007. Characterization of leather industry wastes. Polish Journal of Environmental Studies, 16, 867–873. [Google Scholar]
- Paulsrud B, Gjerde B and Lundar A, 2004. Full scale validation of helminth ova (Ascaris suum) inactivation by different sludge treatment processes. Water Science and Technology. 2004 May; 49, 139–146. [PubMed] [Google Scholar]
- Paluszak Z, Ligocka A and Olszewska H, 2003. Inactivation of Ascaris suum eggs during sewage sludge composting. Medycyna Weterynaryjna, 59, 154–156. [Google Scholar]
- Paluszak Z, Bazeli M, Hermann J and Bauza‐Kaszewska J, 2006. Microbiological investigation of sludge treated with quick lime. Medycyna Weterynaryjna, 62, 1427–1430. [Google Scholar]
- Pan Y, Wang Y, Wang M, Zhang Q, Baloch AR, Zhou J, Ma J, Kashif J, Xu G, Wang L, Fan J, Cui Y and Yu S, 2019. First detection and genetic characterization of ungulate Tetraparvovirus 2 and ungulate Tetraparvovirus 4 in special livestock on the Qinghai‐Tibet Plateau in China. Virology Journal, 16, 56. 10.1186/s12985-019-1167-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson J, Lu X and Gandhi K, 2003. The incineration of wool scouring sludge. Journal of the Textile Institute, 94, 110–118. [Google Scholar]
- Pecson BM and Nelson KL, 2005. Inactivation of Ascaris suum eggs by ammonia. Environmental Science and Technology, 39, 7909–7914. 10.1021/es050659a [DOI] [PubMed] [Google Scholar]
- Pharo HJ, 2002. Foot‐and‐mouth disease: an assessment of the risks facing New Zealand. New Zealand Veterinary Journal, 50, 46–55. 10.1080/00480169.2002.36250 [DOI] [PubMed] [Google Scholar]
- Pokorova D, Vesely T, Piackova V, Reschova S and Hulova J, 2005. Current knowledge on koi herpesvirus (KHV): a review. Veterinární Medicína, 50, 139–148. 10.17221/5607-VETMED [DOI] [Google Scholar]
- Pourcher AM, Devriese L, Hernandez J and Delattre J, 1991. Enumeration by a miniaturized method of Escherichia coli, Streptococcus bovis and enterococci as indicators of the origin of faecal pollution of waters. Journal of Applied Bacteriology, 70, 525–530. 10.1111/j.1365-2672.1991.tb02752.x [DOI] [PubMed] [Google Scholar]
- Qiu J, Söderlund‐Venermo M and Young NS, 2017. Human parvoviruses. Clinical Microbiology Reviews, 30, 43–113. 10.1128/CMR.00040-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadhas A, Jayaraj S and Muraleedharan C, 2005. Biodiesel production from high FFA rubber seed oil. Fuel, 84, 335–340. 10.1016/j.fuel.2004.09.016 [DOI] [Google Scholar]
- Ramos M, Dias APS, Puna JF, Gomes J and Bordado JC, 2019. Biodiesel production processes and sustainable raw materials. Energies, 12, 4408. 10.3390/en12234408 [DOI] [Google Scholar]
- Ryan E, Mackay D and Donaldson A, 2008. Foot‐and‐mouth disease virus concentrations in products of animal origin. Transboundary and Emerging Diseases, 55, 89–98. 10.1111/j.1865-1682.2007.01004.x [DOI] [PubMed] [Google Scholar]
- Sahlström L, 2003. A review of survival of pathogenic bacteria in organic waste used in biogas plants. Bioresource Technology, 87, 161–166. 10.1016/s0960-8524(02)00168-2 [DOI] [PubMed] [Google Scholar]
- Sahlström L, Bagge E, Emmoth E, Holmqvist A, Danielsson‐Tham M‐L and Albihn A, 2008. A laboratory study of survival of selected microorganisms after heat treatment of biowaste used in biogas plants. Bioresource Technology, 99, 7859–7865. 10.1016/j.biortech.2007.09.071 [DOI] [PubMed] [Google Scholar]
- Sai Akhil U and Alagumalai A, 2019. A short review on valorization of slaughterhouse wastes for biodiesel production. ChemistrySelect, 4, 13356–13362. 10.1002/slct.201903739 [DOI] [Google Scholar]
- Saidan M, Khasawneh HJ, Tayyem M and Hawari M, 2017. Getting energy from poultry waste in Jordan: cleaner production approach. Journal of Chemical Technology and Metallurgy, 52, 595–601. [Google Scholar]
- Salminen E and Rintala J, 2002. Anaerobic digestion of organic solid poultry slaughterhouse waste – a review. Bioresource Technology, 83, 13–26. 10.1016/s0960-8524(01)00199-7 [DOI] [PubMed] [Google Scholar]
- Sander A, Antonije Košćak M, Kosir D, Milosavljević N, Parlov Vuković J and Magić L, 2018. The influence of animal fat type and purification conditions on biodiesel quality. Renewable Energy, 118, 752–760. 10.1016/j.renene.2017.11.068 [DOI] [Google Scholar]
- Sargison N, 2009. Sheep Flock Health: A Planned Approach. John Wiley & Sons, 480 pp. [Google Scholar]
- Saucier L and Plamondon É, 2011. Heat inactivation of Mycobacterium avium subsp. paratuberculosis in aseptically prepared ground beef. International Journal of Food Engineering, 77, 1–22. 10.2202/1556-3758.2062 [DOI] [Google Scholar]
- Sauerbrei A and Wutzler P, 2009. Testing thermal resistance of viruses. Archives of Virology, 154, 115–119. 10.1007/s00705-008-0264-x [DOI] [PubMed] [Google Scholar]
- SEAC (Student Environmental Action Coalition), 2003. Fertilisers controls: Paper for SEAC meeting on 24 June 2003. Paper SEAC 78/3. Available online: https://webarchive.nationalarchives.gov.uk/ukgwa/20070308092252/http://www.seac.gov.uk/agenda/agen0603.htm
- Segalés J, Barcellos D, Alfieri A, Burrough E and Marthaler D, 2016. Senecavirus A: an emerging pathogen causing vesicular disease and mortality in pigs? Veterinary Pathology, 54. 10.1177/0300985816653990 [DOI] [PubMed] [Google Scholar]
- Shelef L and Seiter J, 2005. Indirect and miscellaneous antimicrobials. In: Davidson PM, Sofos JN and Branen AL (eds.). Antimicrobials in Food. Taylor and Francis, Boca Raton. pp. 573–598. [Google Scholar]
- Shen Q, Wang H, Zhang C, Qin X, Jia W, Xu X, Richel A and Zheng Q, 2020. Liquefaction of porcine hoof shell to prepare peptone substitute by instant catapult steam explosion. Journal of Bioscience and Bioengineering, 129, 467–475. 10.1016/j.jbiosc.2019.09.019 [DOI] [PubMed] [Google Scholar]
- Shokraneh A, Farhad AR, Farhadi N, Saatchi M and Hasheminia SM, 2014. Antibacterial effect of triantibiotic mixture versus calcium hydroxide in combination with active agents against Enterococcus faecalis biofilm. Dental Materials Journal, 33, 733–738. 10.4012/dmj.2014-090 [DOI] [PubMed] [Google Scholar]
- Singh K, Miller MM, Kohrt LJ, Scherba G, Garrett EF and Fredrickson RL, 2011. Development of a novel diagnostic test for detection of bovine viral diarrhea persistently infected animals using hair. Journal of Veterinary Science, 12, 295–297. 10.4142/jvs.2011.12.3.295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LH, Foster C, Hitchcock ME and Isseroiff R, 1993. In vitro HPV‐11 infection of human foreskin. Journal of Investigative Dermatology, 101, 292–295. 10.1111/1523-1747.ep12365409 [DOI] [PubMed] [Google Scholar]
- Solcova O, Knapek J, Wimmerova L, Vavrova K, Kralik T, Rouskova M, Sabata S and Hanika J, 2021. Environmental aspects and economic evaluation of new green hydrolysis method for waste feather processing. Clean Technologies and Environmental Policy, 23, 1863–1872. 10.1007/s10098-021-02072-5 [DOI] [Google Scholar]
- Sörqvist S, 2003. Heat resistance in liquids of Enterococcus spp., Listeria spp., Escherichia coli, Yersinia enterocolitica, Salmonella spp. and Campylobacter spp. Acta Veterinaria Scandinavica, 44, 1–19. 10.1186/1751-0147-44-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava RN and Lund E, 1980. The stability of bovine parvovirus and its possible use as an indicator for the persistence of enteric viruses. Water Research, 14, 1017–1021. 10.1016/0043-1354(80)90146-3 [DOI] [Google Scholar]
- Strauch D, 1983. German experiences with low and high technology methods for disinfection of municipal sewage sludges. Water Science and Technology, 15, 25–35. 10.2166/wst.1983.0004 [DOI] [Google Scholar]
- Stuckey J, Strauss D, Venkiteshwaran A, Gao J, Luo W, Quertinmont M, O'Donnell S and Chen D, 2014. A novel approach to achieving modular retrovirus clearance for a parvovirus filter. Biotechnology Progress, 30, 79–85. 10.1002/btpr.1820 [DOI] [PubMed] [Google Scholar]
- Syamaladevi RM, Tang J, Villa‐Rojas R, Sablani S, Carter B and Campbell G, 2016. Influence of water activity on thermal resistance of microorganisms in low‐moisture foods: a review. Comprehensive Reviews in Food Science and Food Safety, 15, 353–370. 10.1111/1541-4337.12190 [DOI] [PubMed] [Google Scholar]
- Taylor S and Haldorson G, 2013. A review of equine sarcoid. Equine Veterinary Education, 25, 210–216. 10.1111/j.2042-3292.2012.00411.x [DOI] [Google Scholar]
- Tesfaye T, Sithole B and Ramjugernath D, 2017a. Valorisation of chicken feathers: a review on recycling and recovery route—current status and future prospects. Clean Technologies and Environmental Policy, 19, 2363–2378. 10.1007/s10098-017-1443-9 [DOI] [Google Scholar]
- Tesfaye T, Sithole B, Ramjugernath D and Chunilall V, 2017b. Valorisation of chicken feathers: characterisation of physical properties and morphological structure. Journal of Cleaner Production, 149, 349–365. 10.1016/j.jclepro.2017.02.112 [DOI] [Google Scholar]
- Todd D, 2000. Circoviruses: immunosuppressive threats to avian species: a review. Avian Pathology, 29, 373–394. 10.1080/030794500750047126 [DOI] [PubMed] [Google Scholar]
- Toldrá‐Reig F, Mora L and Toldrá F, 2020. Trends in biodiesel production from animal fat waste. Applied Sciences, 10, 3644. 10.3390/app10103644 [DOI] [Google Scholar]
- Trewby H, Ayele G, Borzacchiello G, Brandt S, Saveria Campo M, Del Fava C, Marais J, Leonardi L, Vanselow B, Biek R and Nasir L, 2014. Analysis of the long control region of bovine papillomavirus type 1 associated with sarcoids in equine hosts indicates multiple cross‐species transmission events and phylogeographical structure. Journal of General Virology, 95, 2748–2756. 10.1099/vir.0.066589-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronina P and Bubel F, 2008. Production of organic fertilizer from poultry feather wastes excluding the composting process. Polish Journal of Chemical Technology, 10, 33–36. 10.2478/v10026-008-0025-3 [DOI] [Google Scholar]
- Tuppurainen ESM and Oura CAL, 2012. Review: lumpy skin disease: an emerging threat to Europe, the Middle East and Asia. Transboundary and Emerging Diseases, 59, 40–48. 10.1111/j.1865-1682.2011.01242.x [DOI] [PubMed] [Google Scholar]
- Tuppurainen ESM, Venter EH, Shisler JL, Gari G, Mekonnen GA, Juleff N, Lyons NA, De Clercq K, Upton C, Bowden TR, Babiuk S and Babiuk LA, 2017. Review: capripoxvirus diseases: current status and opportunities for control. Transboundary and Emerging Diseases, 64, 729–745. 10.1111/tbed.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner C and Williams SM, 1999. Laboratory‐scale inactivation of African swine fever virus and swine vesicular disease virus in pig slurry. Journal of Applied Microbiology, 87, 148–157. 10.1046/j.1365-2672.1999.00802.x [DOI] [PubMed] [Google Scholar]
- Turner C, Williams SM, Burton CH, Farrent JW and Wilkinson PJ, 1998. Laboratory scale inactivation of pig viruses in pig slurry and design of a pilot plant for thermal inactivation. Water Science and Technology, 38, 79–86. 10.1016/S0273-1223(98)00500-9 [DOI] [Google Scholar]
- Turner C, Williams SM, Burton CH, Cumby TR, Wilkinson PJ and Farrent JW, 1999. Pilot scale thermal treatment of pig slurry for the inactivation of animal virus pathogens. Journal of Environmental Science and Health ‐ Part B Pesticides, Food Contaminants, and Agricultural Wastes, 34, 989–1007. 10.1080/03601239909373241 [DOI] [PubMed] [Google Scholar]
- Turner C, Williams SM and Cumby TR, 2000. The inactivation of foot and mouth disease, Aujeszky's disease and classical swine fever viruses in pig slurry. Journal of Applied Microbiology, 89, 760–767. 10.1046/j.1365-2672.2000.01174 [DOI] [PubMed] [Google Scholar]
- Ueda N, Inder MK, Wise LM, Fleming SB and Mercer AA, 2007. Parapoxvirus of red deer in New Zealand encodes a variant of viral vascular endothelial growth factor. Virus research. 2007 Mar 1, 124, 50–58. [DOI] [PubMed] [Google Scholar]
- Ugwuanyi J, Harvey L and McNeil B, 1999. Effect of process temperature, pH and suspended solids content upon pasteurization of a model agricultural waste during thermophilic aerobic digestion. Journal of Applied Microbiology, 87, 387–395. [DOI] [PubMed] [Google Scholar]
- Urlings HA, de Boer GF, van Roozelaar DJ and Koch G, 1993. Inactivation of chicken anaemia virus in chickens by heating and fermentation. Veterinary Quarterly, 15, 85–88. 10.1080/01652176.1993.9694380 [DOI] [PubMed] [Google Scholar]
- USDE (United States Department of Energy), 2020. Biodiesel Production and Distribution. Alternative Fuels Data Center. Available online: https://afdc.energy.gov/fuels/biodiesel_production.html
- USEPA , 1999. Control of pathogens and vector attraction in sewage sludge. Environmental Regulations and Technology, USEPA, Cincinnati, USA. [Google Scholar]
- Valeika V, Beleska K and Sirvaityte J, 2012. Alkali‐free method of hide preparation for tanning. Brazilian Journal of Chemical Engineering, 29, 315–323. 10.1590/S0104-66322012000200012 [DOI] [Google Scholar]
- Van Rogenmortel MH, Fauquet CM, Bishop DH, Carstens EB, Estes MK, Lemon SM, Maniloff J, Mayo MA, McGeoch DJ, Pringle CR and Wickner RB, 2000. Virus Taxonomy: Seventh Report of the International Committee of Taxonomy of Viruses. Academic Press, San Diego, CA, USA. 1162 pp. [Google Scholar]
- Vinnerås B, Björklund A and Jönsson H, 2003. Thermal composting of faecal matter as treatment and possible disinfection method—laboratory‐scale and pilot‐scale studies. Bioresource Technology, 88, 47–54. 10.1016/S0960-8524(02)00268-7 [DOI] [PubMed] [Google Scholar]
- Ward RL and Ashley CS, 1978. Heat inactivation of enteric viruses in dewatered wastewater sludge. Applied and Environmental Microbiology, 36, 898–905. 10.1128/aem.36.6.898-905.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watcharasukarn M, Kaparaju P, Steyer J‐P, Krogfelt KA and Angelidaki I, 2009. Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as indicator organisms in evaluating pathogen‐reducing capacity in biogas plants. Microbial Ecology, 58, 221–230. 10.1007/s00248-009-9497-9 [DOI] [PubMed] [Google Scholar]
- Welch J, Bienek C, Gomperts E and Simmonds P, 2006. Resistance of porcine circovirus and chicken anemia virus to virus inactivation procedures used for blood products. Transfusion, 46, 1951–1958. 10.1111/j.1537-2995.2006.01003.x [DOI] [PubMed] [Google Scholar]
- Wheeler AL, Hartel PG, Godfrey DG, Hill JL and Segars WI, 2002. Potential of Enterococcus faecalis as a human fecal indicator for microbial source tracking. Journal of Environmental Quality, 31, 1286–1293. 10.2134/jeq2002.1286 [DOI] [PubMed] [Google Scholar]
- Williams G, 2017. Persistence of Disease Agents in Carcases and Animal Products. Report for Animal Health Australia by Scott Williams Consulting Pty Ltd., Updated by Herd Health Pty Ltd. [Google Scholar]
- Wilson AJ and Mellor PS, 2009. Bluetongue in Europe: past, present and future. Philosophical Transactions of the Royal Society B: Biological Sciences, 364, 2669–2681. 10.1098/rstb.2009.0091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winfield MD and Groisman EA, 2003. Role of nonhost environments in the lifestyles of Salmonella and Escherichia coli . Applied and Environmental Microbiology, 69, 3687–3694. 10.1128/AEM.69.7.3687-3694.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Wang DK, Kong Y, Ungerfeld EM, Seviour R and Massé DI, 2015. Anaerobic digestibility of beef hooves with swine manure or slaughterhouse sludge. Waste Management, 38, 443–448. 10.1016/j.wasman.2014.12.017 [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K, Yamada M and Mase M, 2010. Persistence of avian influenza virus (H5N1) in feathers detached from bodies of infected domestic ducks. Applied and Environmental Microbiology, 76, 5496–5499. 10.1128/AEM.00563-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto Y, Nakamura K and Mase M, 2017. Survival of highly pathogenic avian influenza H5N1 virus in tissues derived from experimentally infected chickens. Applied and Environmental Microbiology, 83, e00604–e00617. 10.1128/AEM.00604-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B, Wang H, Kaleas K, Butler M, Franklin J, Bill A, Baylis SA, Chen Q and Blümel J, 2020. Clearance of porcine circovirus and porcine parvovirus from porcine‐derived pepsin by low pH inactivation and cation exchange chromatography. Biotechnology Progress, 36. 10.1002/btpr.2968 [DOI] [PubMed] [Google Scholar]
- Yunoki M, Tsujikawa M, Urayama T, Sasaki Y, Morita M, Tanaka H, Hattori S, Takechi K and Ikuta K, 2003. Heat sensitivity of human parvovirus B19. Vox Sanguinis, 84, 164–169. 10.1046/j.1423-0410.2003.00280.x [DOI] [PubMed] [Google Scholar]
- Zhang ZS, Yang DY, Fu YB, Zhang L, Zhao QP and Li G, 2015. Knockdown of CkrL by shRNA deteriorates hypoxia/reoxygenation‐induced H9C2 cardiomyocyte apoptosis and survival inhibition Via Bax and downregulation of P‐Erk1/2. Cell Biochemistry and Function, 33, 80–88. 10.1002/cbf.3093 [DOI] [PubMed] [Google Scholar]
- Zhou T, Jia H, Chen G, He X, Fang Y, Wang X, Guan Q, Zeng S, Cui Q and Jing Z, 2012. Phylogenetic analysis of Chinese sheeppox and goatpox virus isolates. Virology Journal, 9, 25. 10.1186/1743-422X-9-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccola M, Montarsolo A, Mossotti R, Patrucco A and Tonin C, 2015. Green hydrolysis as an emerging technology to turn wool waste into organic nitrogen fertilizer. Waste and Biomass Valorization, 6, 891–897. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Protocol


