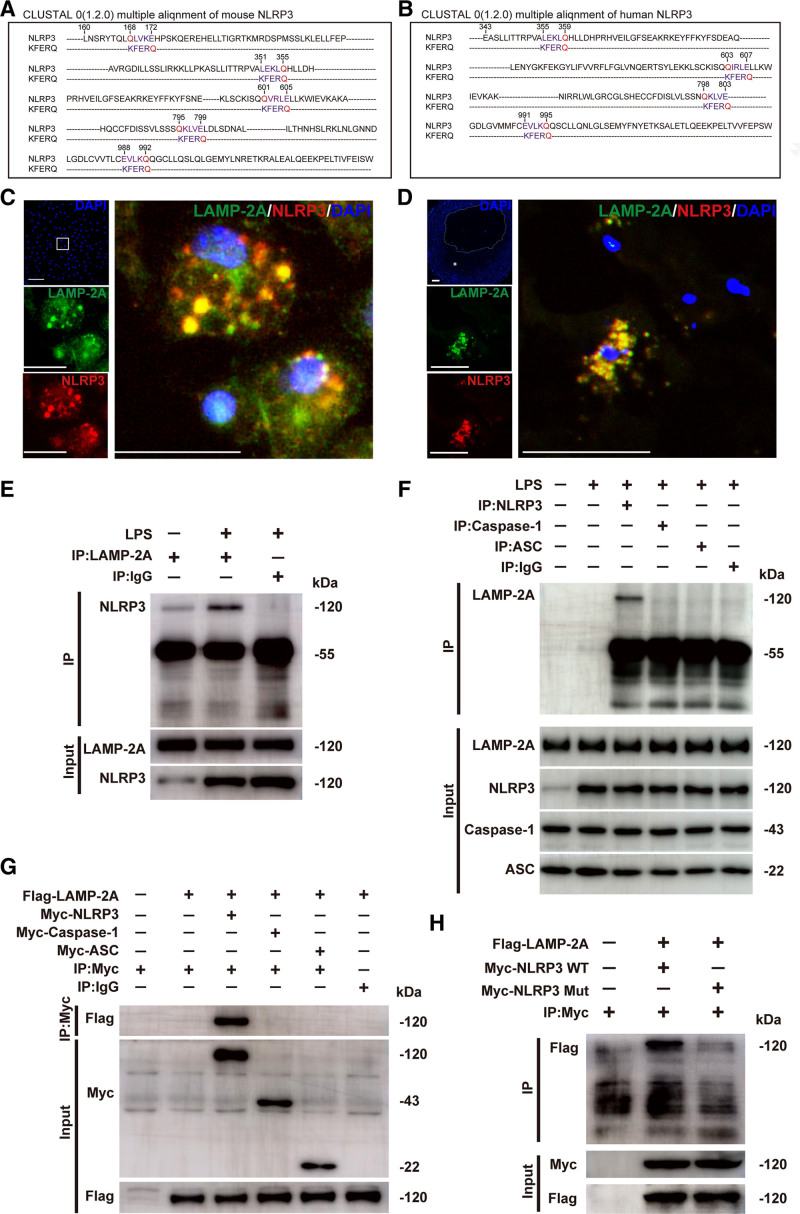
Figure 6.
Identification of the NLRP3 (NLR [NOD-like receptor] family, pyrin domain containing 3) protein as a chaperone-mediated autophagy (CMA) substrate. A and B, Alignment of the amino acid sequences of mouse and human NLRP3 with the KFERQ motif. Within the KFERQ motifs, Q (in red) and the other 4 amino acids (in purple) were marked. C, Colocalization (yellow particles) of NLRP3 (red particles) with LAMP-2A (lysosome-associated membrane protein type 2A; green particles) in peritoneal macrophages treated with lipopolysaccharide (LPS; 100 ng/mL) for 8 h, followed by stimulation with ATP (5 mmol/L) for 30 min. Scale bar=10 μm. D, Colocalization (yellow particles) of NLRP3 (red particles) with LAMP-2A (green particles) in human coronary atherosclerotic plaques. Five independent experiments were performed. Scale bar=100 μm. E, Co-immunoprecipitation of endogenous LAMP-2A with NLRP3 from LPS and ATP-treated mouse peritoneal macrophages. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. F, Co-immunoprecipitation of endogenous NLRP3, caspase-1, or ASC (apoptosis-associated speck-like protein containing a CARD [C-terminal caspase-recruitment domain]) with LAMP-2A from LPS and ATP-treated mouse peritoneal macrophages. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. G, Co-immunoprecipitation of Flag-LAMP-2A with Myc (Myc epitope tag)-NLRP3, Myc-Caspase-1, or Myc-ASC in HEK293T cells. The same amount of nonspecific antibody (Rabbit IgG) was used as a control. H, Co-immunoprecipitation of Flag-LAMP-2A with wild-type (WT) Myc-NLRP3 (Myc-NLRP3 WT) or mutant Myc-NLRP3 (Myc-NLRP3 Mut) from HEK293T cells. All of the four KFERQ motifs in human NLRP3 were mutated to disrupt the interaction of the NLRP3 protein with the CMA receptor LAMP-2A. DAPI indicates 4,6-diamidino-2-phenyiindole; and mut, mutant.