Abstract
Pathogenic Leptospira are the causative agents of leptospirosis, the most widespread zoonotic infectious disease. Leptospirosis is a potentially severe and life-threatening emerging disease with highest burden in sub-tropical areas and impoverished populations. Mechanisms allowing pathogenic Leptospira to survive inside a host and induce acute leptospirosis are not fully understood. The ability to resist deadly oxidants produced by the host during infection is pivotal for Leptospira virulence. We have previously shown that genes encoding defenses against oxidants in L. interrogans are repressed by PerRA (encoded by LIMLP_10155), a peroxide stress regulator of the Fur family. In this study, we describe the identification and characterization of another putative PerR-like regulator (LIMLP_05620) in L. interrogans. Protein sequence and phylogenetic analyses indicated that LIMLP_05620 displayed all the canonical PerR amino acid residues and is restricted to pathogenic Leptospira clades. We therefore named this PerR-like regulator PerRB. In L. interrogans, the PerRB regulon is distinct from that of PerRA. While a perRA mutant had a greater tolerance to peroxide, inactivating perRB led to a higher tolerance to superoxide, suggesting that these two regulators have a distinct function in the adaptation of L. interrogans to oxidative stress. The concomitant inactivation of perRA and perRB resulted in a higher tolerance to both peroxide and superoxide and, unlike the single mutants, a double perRAperRB mutant was avirulent. Interestingly, this correlated with major changes in gene and non-coding RNA expression. Notably, several virulence-associated genes (clpB, ligA/B, and lvrAB) were repressed. By obtaining a double mutant in a pathogenic Leptospira strain, our study has uncovered an interplay of two PerRs in the adaptation of Leptospira to oxidative stress with a putative role in virulence and pathogenicity, most likely through the transcriptional control of a complex regulatory network.
Author summary
Leptospirosis is a widespread infectious disease responsible for over one million of severe cases and 60 000 fatalities annually worldwide. This neglected and emerging disease has a worldwide distribution, but it mostly affects populations from developing countries in sub-tropical areas. The causative agents of leptospirosis are pathogenic bacterial Leptospira spp. There is a considerable deficit in our knowledge of these atypical bacteria, including their virulence mechanisms. In addition to the Leptospira PerRA regulator that represses defenses against peroxide, we have identified and characterized a second PerR regulator in pathogenic Leptospira species (PerRB) that participates in Leptospira tolerance to superoxide. Phenotypic and transcriptomic analyses of single PerRA and PerRB mutants suggest that the two PerRs fulfill distinct functions in the adaptation to oxidative stress. Concomitant inactivation of PerRA and PerRB resulted in a higher tolerance to both peroxide and superoxide. Moreover, the perRAperRB mutant lost its virulence. Major changes in gene expression, including a decreased expression of several virulence factors, were observed in the double perRAperRB mutant. Our study suggests that PerRA and PerRB cooperate to orchestrate a complex regulatory network involved in Leptospira virulence.
Introduction
Pathogenic Leptospira spp. are aerobic diderm bacteria of the spirochetal phylum that are the causative agents of leptospirosis, the most widespread zoonosis [1]. More than one million cases of leptospirosis are currently estimated annually in the world, with about 59000 deaths [2]. This disease is considered as a health threat among impoverished populations in developing countries under tropical areas [2], but the number of reported cases of leptospirosis are also on the rise in developed countries under temperate climates [3]. Rodents are asymptomatic reservoir for leptospires as the bacteria colonize the proximal renal tubules of these animals. Leptospires are shed in the environment through the urine of infected animals and leptospirosis is transmitted to other animal species and humans mostly by exposure to contaminated soils and water. Leptospira penetrate an organism through abraded skins and mucous membranes, subsequently disseminate within the bloodstream and rapidly spread to multiple tissues and organs (including kidney, liver, lungs). Clinical manifestations range from a mild flu-like febrile state to more severe and fatal cases leading to hemorrhages and multiple organ failure. Because of the lack of efficient tools for genetic manipulation of Leptospira spp. and their fastidious growth in the laboratory conditions, our understanding of the mechanism of pathogenicity and virulence as well as the basic biology of these pathogens have been greatly hampered [4,5].
During their life cycle, most bacteria will be exposed to reactive oxygen species (ROS), such as superoxide anion (•O2-) and hydrogen peroxide (H2O2), that are produced endogenously through the aerobic respiratory chain or encountered in the environment [6]. These ROS are also produced, together with hypochlorous acid (HOCl) and nitric oxide anion (•NO), as powerful and efficient weapons by eukaryotic innate immune cells upon infection by pathogenic bacteria [7]. ROS cause oxidative damage to cellular components (proteins, DNA and lipids) and this would result in bacterial death if bacteria had not developed scavenging enzymes to counteract the deadly effect of ROS, including catalase, peroxidases and superoxide dismutase or reductase (SOD, SOR).
ROS production is increased in human and animals upon infection by Leptospira [8–10]. In fact, the ability to detoxify H2O2 is essential for Leptospira virulence as inactivation of the catalase-encoding gene led to virulence attenuation in L. interrogans [11]. The oxidative stress response in pathogenic Leptospira spp. is controlled by the ROS sensing transcription factor peroxide stress regulator (PerR) (encoded by LIMLP_10155/LIC12034/LA1857) [12,13]. PerR belongs to the Fur (Ferric uptake regulator) transcriptional factor family. It is a metalloprotein that binds DNA in the presence of iron or manganese and represses the expression of catalase and peroxidases [14–16]. In the presence of peroxide, iron-bound PerR is oxidized on the histidine residues participating in iron coordination. Iron is released and PerR dissociates from DNA. As a consequence, peroxide-scavenging enzyme repression is alleviated [17,18].
We have very recently characterized the transcriptional response to hydrogen peroxide in the pathogen L. interrogans and shown that these bacteria respond to sublethal H2O2 concentration by increasing the expression of catalase and of two peroxidases (Alkylperoxiredoxin (AhpC) and Cytochrome C peroxidase (CCP)) [19]. When Leptospira were exposed to deadly H2O2 concentration, additional enzymes with a putative role as antioxidants and/or in repair of oxidized cysteines in proteins were up-regulated, including several thiol oxidoreductases (thioredoxin, glutaredoxin, DsbD, and Bcp-like proteins) [19]. Several genes of the LexA regulon (recA, recN, dinP) and other genes with putative role in DNA repair (mutS, radC) had a higher expression as well as genes encoding for canonical chaperones (dnaK/dnaJ/grpE, groES/EL, and hsp15/20) [19]. Only genes coding for the catalase and peroxidases were under the control of PerR and our study has revealed a complex regulatory network independent of PerR involving other transcriptional regulators, sigma factors, two component systems and non-coding RNAs [19]. During the course of this study, we noticed that an ORF encoding a Fur-like regulator (LIMLP_05620/LIC11158/LA2887) was up-regulated when Leptospira were exposed to a deadly concentration of H2O2.
Here, we report the functional characterization of the LIMLP_05620-encoding Fur-like regulator (PerRB) in the adaptation of Leptospira to oxidative stress. In silico analyses demonstrate that PerRB exhibits PerR canonical amino acid residues and is the closest relative of PerRA (encoded by LIMLP_10155). Furthermore, a perRB mutant has a higher tolerance to superoxide. By obtaining a double mutant where perRA and perRB are concomitantly inactivated, we have also investigated the interplay between these two PerR-like regulators in the adaptation to oxidative stress and virulence of L. interrogans. Our study suggests that LIMLP_05620 encodes a second PerR-like regulator in pathogenic Leptospira species that putatively cooperates with PerRA in controlling Leptospira virulence.
Results
Identification of an ORF that encodes a novel putative PerR regulator in pathogenic Leptospira species
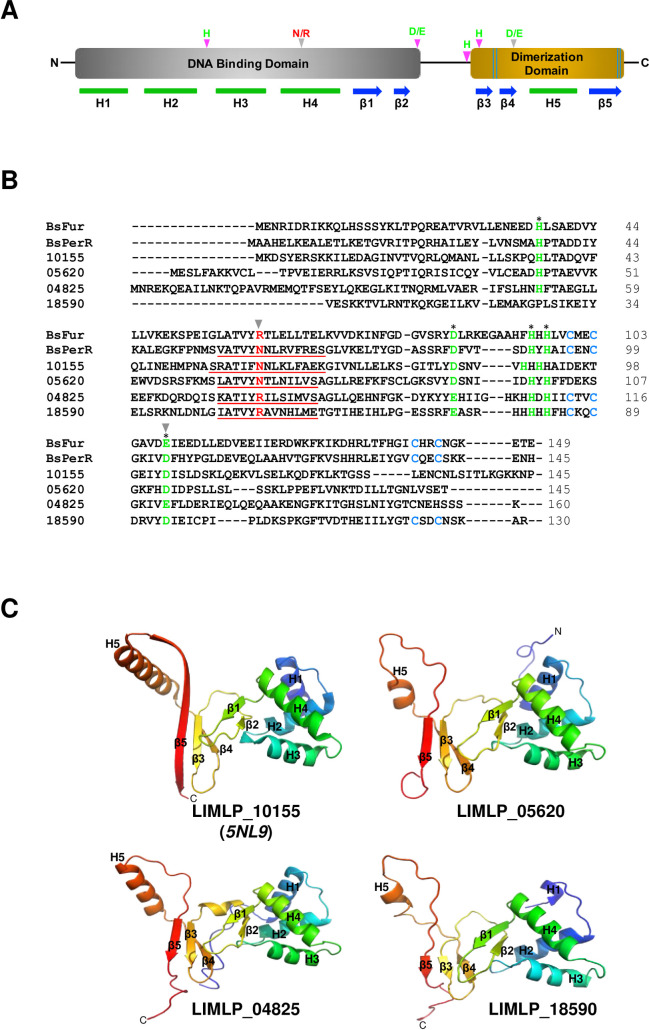
Regulators of the Fur family are homodimeric metalloproteins with a two-domain organization composed of an amino-terminal DNA binding domain and a carboxy-terminal dimerization domain (Fig 1A). The DNA binding domain contains a winged helix-turn-helix (HTH) DNA binding motif (H2-H4, in Fig 1A) where the H4 helix mediates DNA binding. The dimerization domain consists of an α/β domain. The regulatory iron that controls DNA binding and dissociation is coordinated by histidine, aspartate, and glutamate residues located in a loop at the hinge of the amino and carboxy-terminal domains. Most of Fur-like regulators also coordinate a structural metal (zinc) through 2 cysteinate motifs (CxxC, where x designates any AA). This structural metal allows correct folding and dimeric assembly of the regulator.
Fig 1. Analysis of the four Fur-like regulators of L. interrogans.
(A) Schematic representation of the domain organization of a typical Fur-like regulator. The N-terminal DNA binding domain and the C-terminal dimerization domain are represented in grey and golden, respectively. The α-helix and β-strand secondary structures are indicated below in green and blue, respectively. The His, Asp and Glu residues involved in regulatory metal coordination are designated in green. The Arg/Asn residue involved in DNA binding specificity is marked in red. The Arg/Asn (involved in DNA binding specificity) and Asp/Glu residues (involved in H2O2 sensitivity) that allow distinguishing a Fur from a PerR are further emphasized with a grey arrow head. The two cysteinate motifs in the C-terminal domain involved in structural metal coordination are represented by the double blue lines in the C-terminal dimerization domain. (B) Comparison of the four Fur-like regulators of L. interrogans (LIMLP_10155, LIMLP_05620, LIMLP_04825, LIMLP_18590) with B. subtilis Fur and PerR. Primary sequence alignment was obtained by Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/; [70]). The H4 DNA binding helix is underlined and the Arg/Asn residue involved in DNA binding specificity is designated in red. The residues of the regulatory metal coordination, including the Asp/Glu residue involved in H2O2 sensitivity, are marked in green and indicated with an asterisk. The Arg/Asn and Asp/Glu residues that allow distinguishing a Fur from a PerR are further emphasized with a grey arrow head. The cysteine residues of the structural metal coordination are marked in cyan. (C) Cartoon representation of the crystal structure of LIMLP_10155 (5NL9) and of the modeled structure of LIMLP_05620, LIMLP_04825 and LIMLP_18590. The modeled structures were obtained by searching homologies between LIMLP_05620, LIMLP_04825 and LIMLP_18590 and proteins with known crystal structure using PHYRE2 (http://www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index; [71]). Secondary structures are numbered as in (A).
Due to high conservation in folding, metal coordination and similarity in the metal-induced conformational switch controlling DNA binding, it is difficult to distinguish members of the Fur family on the sole basis of their primary sequence. However, in B. subtilis, a single amino acid residue in the H4 helix of the amino-terminal DNA binding domain (N61 and R61 for B. subtilis PerR and Fur, respectively) allows PerR and Fur to discriminate between their respective DNA sequence targets (PerR and Fur boxes, respectively) [20] (Fig 1B). In addition, D104 residue in the carboxy-terminal domain is pivotal in the PerR sensitivity to H2O2. The corresponding residue in Fur is a glutamate and mutating this residue to aspartate leads to H2O2 sensitivity [21] (Fig 1B). Therefore, N61 and D104, which are well-conserved in other bacterial species, are considered as canonical PerR amino acid residues [20,21].
L. interrogans genome encodes 4 ORFs that share homology with regulators of the Fur family. Sequence alignment of these ORFs with the Fur and PerR from B. subtilis shows a good conservation of residues involved in the regulatory metal coordination (Fig 1B). Interestingly, two of the 4 Fur-like ORFs of L. interrogans, LIMLP_10155 (LIC12034/LA1857 encoding a PerR) and LIMLP_05620 (LIC11158/LA2887), exhibit the canonical asparagine (N60 and N68, respectively) and aspartate (D103 and D112, respectively) residues of a typical PerR. The third ORF encoding a putative Fur-like regulator, LIMLP_04825 (LIC11006/LA3094), possesses the two Fur typical residues, R76 and E121, respectively. The fourth ORF encoding a putative Fur-like regulator, LIMLP_18590 (LIC20147/LB183), possesses the typical Fur arginine residue in its putative H4 DNA binding helix (R51) but a typical PerR aspartate residue in the carboxy-terminal domain (D94). Of note, LIMLP_18590 has a glutamate residue at the position 96. Fold prediction suggests that the three Fur regulators encoded by LIMLP_05620, LIMLP_04825 and LIMLP_18590 adopt the two-domain organization typical of the Fur family depicted in the crystal structure of LIMLP_10155 (Fig 1C).
The closest relative of the PerR-encoding LIMLP_10155 is LIMLP_05620 with about 26% of sequence identity, and LIMLP_04825 and LIMLP_18590 are closest relatives that share 20% of sequence identity. LIMLP_05620 shares about 27% identity with the well-characterized B. subtilis PerR. The putative H4 helix in LIMLP_05620 (Leu63-Ser75) is relatively well conserved with that of B. subtilis (Val56-Ser69) (Fig 1B and 1C) and LIMLP_05620 displays a typical regulatory metal coordination site (His44-Asp93-His99-His101-Asp112). As the LIMLP_10155-encoded PerR, LIMLP_05620 lacks the cysteinate motif involved in structural metal coordination [13] (Fig 1B). In contrast, both LIMLP_04825 and LIMLP_18590 have one or two cysteinate motifs for structural metal coordination (C113xxC116 and C86xxC89-C122xxC125, respectively). Therefore, LIMLP_05620 encodes a putative PerR-like regulator closely related to the PerR-encoding LIMLP_10155 whereas LIMLP_04825 and LIMLP_18590 could encode other type of Fur-like regulators (Fur, Zur, or Nur). We therefore annotated the LIMLP_10155 and LIMLP_05620 ORFs as perRA and perRB, respectively.
Phylogenetic analysis of PerRA and PerRB in Leptospira species
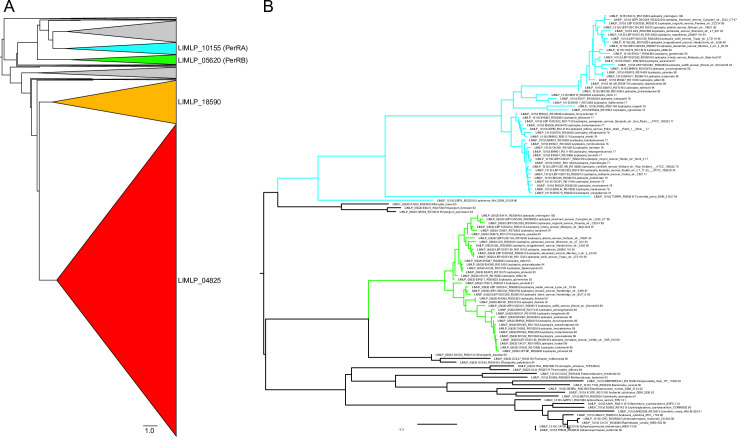
To get a better understanding of the evolutionary relationship of the four Fur-like regulators in pathogenic Leptospira, we undertook phylogenetic analyses by searching for homologous sequences of the PerRA (LIMLP_10155), PerRB (LIMLP_05620), LIMLP_18590 and LIMLP_04825 proteins among the representative genomes present in GenBank. This revealed a large phylogenetic distribution with several branches (Fig 2A). The sequences homologous to the LIMLP_04825 and LIMLP_18590 proteins form two distinct groups (red and orange, respectively) separated by a common ancestor. To get better definition of phylogenetic relationships of PerR-like homologues, we performed analysis with only a subset of sequence (Fig 2B). This phylogenetic analysis shows two separated groups composed of the sequences of PerRA (LIMLP_10155) and PerRB (LIMLP_05620) (see S1 Fig for a more complete and detailed tree).
Fig 2.
(A) Phylogenetic tree with a cartoon representation showing the distribution of the 1671 sequences putatively homologous to the PerRA (LIMLP_10155, cyan triangle), PerRB (LIMLP_05620, green triangle), LIMLP_18590 (yellow triangle) and LIMLP_04825 (red triangle) proteins. The gray triangles represent groups which are not monophyletic with the Leptospira sequences and which may therefore originate from other types of PerR or have had a species-specific evolution. (B) Phylogenetic tree showing the separation between PerRA (cyan) and PerRB (green).
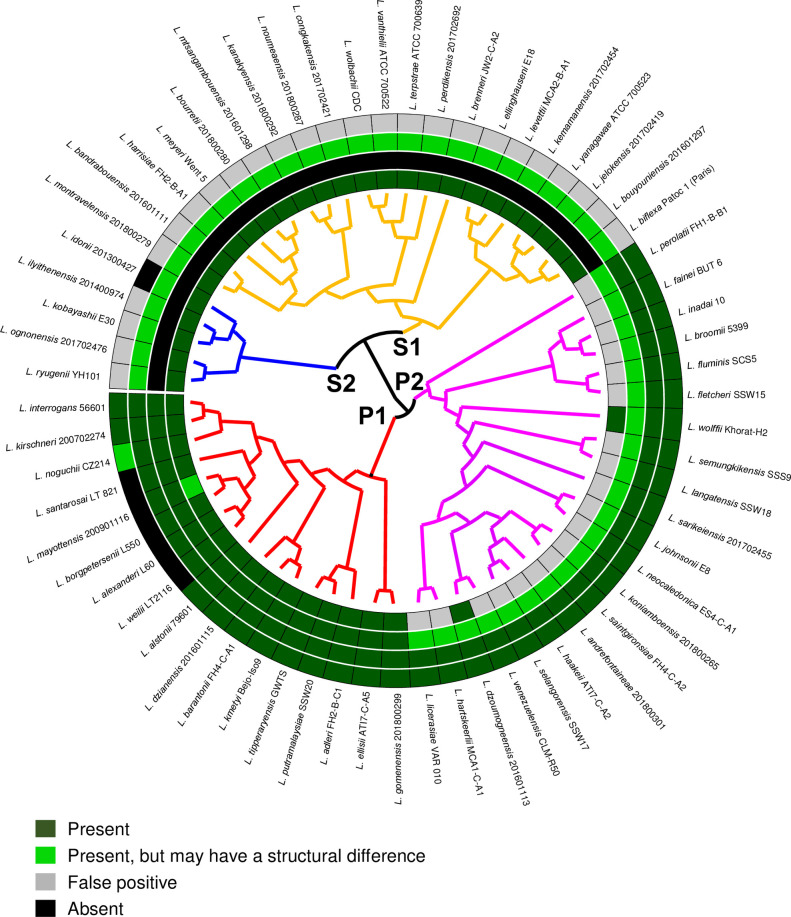
The sequences of PerRA (LIMLP_10155) and PerRB (LIMLP_05620) ORFs from the strain L. interrogans serovar Manilae were searched and compared in all available genomes from the Leptospira genus (S1 Table). As seen in Fig 3, PerRA is present in the saprophytes S1 and S2 clades and in the P1 clade (highly virulent strains). This ORF is absent from most of the P2 clade (intermediate strains). However, there are two exceptions in the P2 clade species since homologues of PerRA are present in L. dzoumogneensis and L. wolffii. Additionally, this ORF is also present in other bacteria from the order Leptospirales such as Turneriella parva and Leptonema illini (Fig 2B). This suggests that PerRA was present in Leptospirales ancestor before Leptospira divergence and lost in the P2 clade. On the other side, PerRB ORF is only present in P1 and P2 clades and absent in all species from S1 and S2 clades (Fig 3). PerRB is also not found in other bacteria from the Leptospirales order (Figs 2B and S1). This restricted distribution suggests that the ancestor of pathogenic strains (P1 and P2 clades) has likely acquired PerRB after their divergence with other Leptospira. Overall, both PerRA and PerRB ORFs only coexist in P1 clade that comprises the highly virulent Leptospira species. Altogether, these findings indicate that pathogenic Leptospira strains encode a second putative PerR-like regulator that is absent in saprophytes.
Fig 3. Distribution of the four Fur-like regulators of L. interrogans in the genus Leptospira.
Circular phylogenetic tree with inner circles indicating the homology between each Fur-like regulator of L. interrogans with the closest homolog among representative genomes of Leptospira species. The branches are colored according to their classification into the four main subclades with P1 (highly pathogenic) in red, P2 (intermediates) in magenta, S1 (saprophytes) in yellow and S2 (new clade saprophytes) in blue [58]. The inner circles are, from the inside to the outside, PerRA (LIMLP_10155), PerRB (LIMLP_05620), LIMLP_04825 and LIMLP_18590. The green color gradient indicates the degree of homology (See S1 Table). Grey indicates the presence of a false positive (a different fur-like regulator with low homology) and black indicates the absence of orthologs.
PerRB is involved in L. interrogans tolerance to ROS
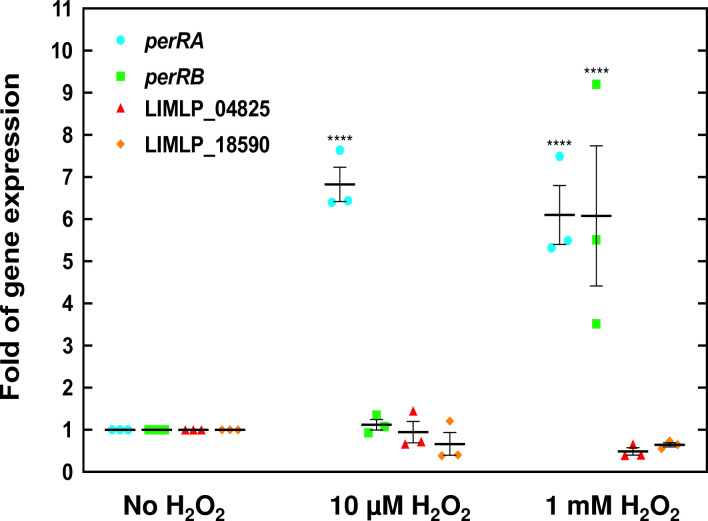
As demonstrated previously [19], when L. interrogans are exposed to a subtlethal dose of H2O2 (10 μM for 30 min) perRA expression is increased by a 7-fold whereas that of perRB is unchanged (Fig 4). In the presence of a higher dose of H2O2 (1 mM for 1h), expression of both perRA and perRB was increased significantly by a 6-fold (Fig 4). Interestingly, the expression of the genes encoding the two other Fur-like regulators (LIMLP_04825 and LIMLP_18590) was not changed in the presence of H2O2. This indicates that PerRA and PerRB are the only Fur-like regulators that respond to H2O2 in L. interrogans.
Fig 4. Increased perRA and perRB expression upon exposure to hydrogen peroxide.
Exponentially growing L. interrogans were incubated in the absence or presence of 10 μM (for 30 min.) or 1 mM H2O2 (for 60 min.) and the expression of perRA (cyan circles), perRB (green squares), LIMLP_04825 (red triangles) and LIMLP_18590 (orange diamonds) was measured by RT-qPCR as described in the Materials and Methods section using flaB as reference gene. Gene expression was normalized by that in untreated samples. Data are the means and standard errors of three independent biological replicates. Two-way Anova test indicated statistical significance in comparison with untreated samples (****, p-values<0.0001).
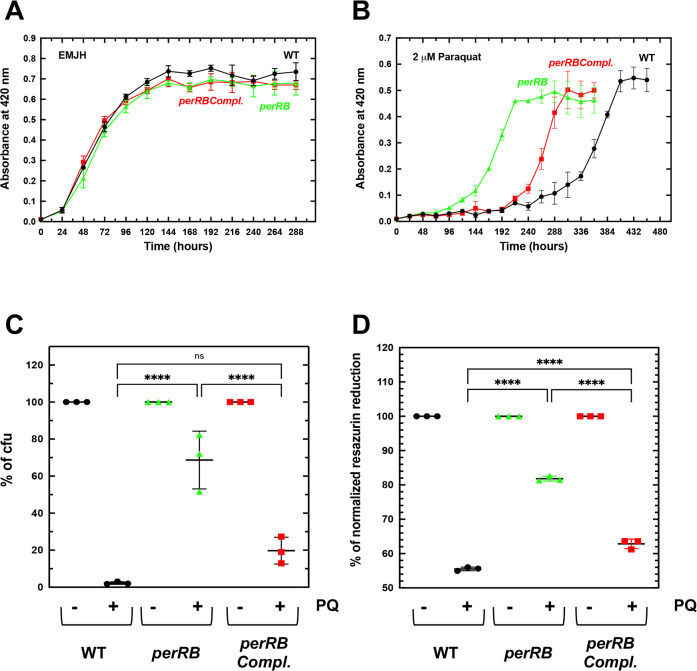
We have previously shown that inactivating perRA led to the derepression of katE, ahpC and ccp and to a higher tolerance to H2O2 [13,19] (see S2 Fig). In addition, the perRA mutant exhibited a reduced ability to grow in the presence of the superoxide-generating compound paraquat [13]. A mutant with a transposon inserted into the PerRB-encoding LIMLP_05620 ORF was available in our random transposon mutant library and was used to investigate the role of PerRB in L. interrogans tolerance to ROS. Inactivating perRB did not have any effect on the ability of L. interrogans to tolerate deadly concentration of H2O2 (S2 Fig); however, it increases the capability of Leptospira to grow in the presence of paraquat (Fig 5B). Indeed, logarithmic growth of WT started after about 12 days in the presence of paraquat whereas that of the perRB mutant began after 5 days. Survival upon 1h exposure to 100 μM paraquat, as measured by colony-forming unit, showed that the perRB mutant had a 34-fold higher survival than that of the WT strain (Fig 5C). Resazurin reduction assay also confirmed the higher survival of the perRB mutant in the presence of paraquat (Fig 5D). Complementing in trans the perRB mutant (S3 Fig) partially restored the WT growth and survival phenotypes in the presence of paraquat (Fig 5B and 5D). Indeed, logarithmic growth of the complemented strain started after 8–9 days and its survival decreased to a 9-fold higher value than that of the WT strain (Fig 5B and 5C). This indicates that PerRB is involved in L. interrogans tolerance to superoxide.
Fig 5. Effect of perRB inactivation on Leptospira growth and survival in the presence of superoxide-generating paraquat.
L. interrogans WT containing the empty pMaORI vector (black circles), the perRB mutant containing the empty pMaORI vector (green triangles) or the perRB mutant containing the pMaORI vector expressing LIMLP_05620 (red squares) were cultivated in EMJH medium complemented with spectinomycin in the absence (A) or in the presence of 2 μM Paraquat (B). Growth was assessed by measure of absorbance at 420 nm. Data are means and standard errors of four independent biological replicates. In panel B, two-Anova test indicated that the differences between the perRB mutant and the WT were statistically significant from 144 to 360 h (p-value<0.0001), the differences between the perRB mutant and the complemented strain were statistically significant from 168 to 264 h (p-value<0.0001), and the differences between the complemented strain and the WT were statistically significant from 264 to 360 h (p-value<0.0001). Quantitative survival tests were performed by incubating exponentially growing WT containing the empty pMaORI vector (black circles), the perRB mutant containing the empty pMaORI vector (green triangles) or the perRB mutant containing the pMaORI vector expressing LIMLP_05620 (red squares) with 100 μM paraquat for 60 min. Percent of colony-forming unit (cfu) (C) and resazurin reduction (D) were determined as described in the Materials and Methods section. Results are shown as mean and SD of three independent experiments. Statistical significance was determined by a One-way Anova test (****, p-value<0.0001; ns, non-significant).
Identification of differentially-expressed genes upon perRB inactivation
To understand the role of PerRB in L. interrogans tolerance to ROS, we compared the global transcriptional profiles of the perRB mutant and WT strains. Differential gene expression analysis revealed changes in the transcription of 123 genes, with 59 and 64 down- and up-regulated, respectively (see S2 Table for a complete set of data). However, perRB inactivation did not lead to dramatic changes in gene expression as the majority of Log2FC (108 out of 123) ranged between -1 and 1 (S2 Table). These findings indicate that the absence of an active PerRB did not lead to substantial significant changes in genes expression when Leptospira are cultivated in the laboratory conditions (in EMJH medium at 30°C) and during the exponential phase.
Nevertheless, when examining the nature of the highest differentially-expressed genes in the perRB mutant, some tendencies could be observed. Many of the differentially-expressed ORFs were annotated as encoding proteins with unknown function and did not have homologs in the saprophyte L. biflexa strain (Tables S2 and 1).
Table 1. Differentially-expressed ORFs upon perRB inactivation.
| ORF IDa | Gene | L. biflexab | Function | Log2FC | Adjusted p-value |
|---|---|---|---|---|---|
| Down-regulated genes | |||||
| LIMLP_02490* (LIC12988/LA0587) | LEPBI_I0886 | Lipase putative extracellular lipoprotein | -1.608 | 6.11e-04 | |
| LIMLP_02845 (LIC12920) | Hypothetical | -1.084 | 3.46e-02 | ||
| LIMLP_03640** (LIC12763/LA0865) | Hypothetical | -1.102 | 1.06e-02 | ||
| LIMLP_03790* (LIC12736/LA0905) | Hypothetical | -1.244 | 1.42e-05 | ||
| LIMLP_04255 (LIC10892/LA3244) | exbB | LEPBI_I0149 | Biopolymer transport protein ExbB/TolQ | -0.947 | 1.07e-02 |
| LIMLP_06190** (LIC11265/LA2751) | LEPBI_I3113 | Disulfide oxidoreductase | -0.723 | 6.96e-03 | |
| LIMLP_11400** (LIC12297/LA1456) | radC | DNA repair protein RadC | -0.619 | 1.17e-02 | |
| LIMLP_13165** (LIC12631/LA1029) | sph2 | Sphingomyelinase C | -1.152 | 8.10e-03 | |
| LIMLP_14595* (LIC10628/LA3571) | LEPBI_I2694 | Cytochrome oxidase CcoP subunit | -0.583 | 3.40e-02 | |
| LIMLP_14715** (LIC10606/LA3598) | dps | LEPBI_I2540 | DNA-binding stress protein Dps | -0.896 | 1.61e-03 |
| LIMLP_15470** (LIC10454/LA3793) | LEPBI_I0671 | Hemolysin (N-acyltransferase domain) | -1.317 | 3.40e-03 | |
| LIMLP_15890 (LIC10377/LA0430) | Hypothetical putative lipoprotein | -1.353 | 3.90e-10 | ||
| LIMLP_16695 (LEPIC3326/LA4096) | Hypothetical | -1.124 | 8.14e-04 | ||
| LIMLP_18235** (LIC20078/LB099) | Hypothetical | -1.098 | 1.69e-02 | ||
| Up-regulated genes | |||||
| LIMLP_02880* (LIC12912/LA0688) | cas5 | CRISPR-associated protein Cas5 | 0.881 | 5.84e-03 | |
| LIMLP_02885* (LIC12911-12910/LA0689-0690) | cas3 | CRISPR-associated protein Cas3 | 0.834 | 4.51e-03 | |
| LIMLP_04075** (LIC12680/LA0974) | Adhesin/FimH-like protein/DuF1566 domain | 1.110 | 4.29e-03 | ||
| LIMLP_05450* (LIC11125/LA2933) | Diguanylate cyclase | 0.983 | 2.12e-04 | ||
| LIMLP_05455* (LIC11126/LA2932) | Diguanylate cyclase | 0.757 | 1.81e-02 | ||
| LIMLP_05460* (LIC11127/LA2930) | Diguanylate cyclase | 0.922 | 1.11e-03 | ||
| LIMLP_05480 (LA2928) | Hypothetical | 0.968 | 2.67e-02 | ||
| LIMLP_05485** (LIC11131/LA2926) | Diguanylate cyclase | 0.679 | 8.14e-04 | ||
| LIMLP_05845 (LIC11203/LA2827) | Diguanylate phosphodiesterase | 0.608 | 3.85e-02 | ||
| LIMLP_09580 (LIC11921/LA1980) | LEPBI_I1269 | Diguanylate phosphodiesterase | 0.547 | 1.61e-02 | |
| LIMLP_17875 (LIC20015/LB017) | hemN | LEPBI_I1166 | Coproporphyrinogen III oxidase HemN | 0.727 | 9.53e-03 |
| LIMLP_18375** (LIC20106/LB133) | Diguanylate phosphodiesterase | 0.593 | 1.99e-02 | ||
| LIMLP_18755 (LIC20176/LB225) | Hypothetical putative lipoprotein | 1.453 | 3.77e-04 | ||
| LIMLP_18760 (LIC20177/LB226) | Adhesin/FimH-like protein/ DUF1566 domain-containing protein | 1.095 | 3.39e-02 | ||
| LIMLP_19320* (LA1770) | AraC family transcriptional regulator | 1.279 | 4.51e-03 | ||
| LIMLP_19325* (LA1771) | Hypothetical | 1.262 | 4.05e-02 |
Selected up-and down-regulated genes in the perRB mutant with an adjusted p-value cutoff of 0.05.
a Gene numbering is according to Satou et al. [22]. Corresponding genes of L. interrogans serovar Lai strain 56601 and serovar Copenhageni Fiocruz strain L1-130 are indicated in parenthesis.
b Closest ortholog in the saprophytes L. biflexa serovar Patoc strain Patoc1. The absence of synteny is indicated in italic.
Genes that are down-regulated upon perRA inactivation as determined previously [19] are indicated in bold.
Down (*) and up (**) -regulated genes upon exposure to lethal H2O2 dose as determined previously [19].
Several genes involved in the metabolism of c-di GMP were differentially-expressed upon perRB inactivation. C-di GMP is a secondary messenger in bacteria that regulates a variety of processes such as biofilm formation, motility, stress adaptation, and virulence. C-di GMP synthesis is catalyzed by diguanylate cyclases (DGCs) whereas its hydrolysis is catalyzed by phosphodiesterases (PDEs). DGCs and PDEs are numerous in pathogenic Leptospira, suggesting that c-di GMP fulfills an important role in sensing environmental signals when Leptospira infect and colonize a host. C-di GMP has been recently shown to regulate biofilm formation, motility and protection against environmental stress in pathogenic Leptospira [23]. Four DGCs (LIMLP_05450, LIMLP_05455, LIMLP_05460, LIMLP_05485) were up-regulated upon perRB inactivation (Table 1). These DGC-encoding ORFs are located in a gene cluster (LIMLP_05485–05450) that contains 7 ORFs coding for DGCs. LIMLP_05450, LIMLP_05455, LIMLP_05460, and LIMLP_05485 display the typical diguanylate cyclase GGDEF and sensory PAS domains. A DGC activity was demonstrated in vitro for LIMLP_05450, LIMLP_05455, LIMLP_05460 [24]. Three PDE-encoding ORFs (LIMLP_05845, LIMLP_9580, and LIMLP_18375) were also up-regulated in the perRB mutant.
Among the highest up-regulated genes, two ORFs (LIMLP_04075 and LIMLP_18760) encoded lipoproteins with a putative adhesin function. These proteins contain DUF1566 domain repeats which is also share by Lsa25, a Leptospiral surface adhesin that binds extracellular matrix (ECM) [25].
An ORF encoding an AraC transcriptional regulator (LIMLP_19320), and two ORFs of unknown function (LIMLP_18755 and 19325) were also among the most up-regulated. The orthologs of LIMLP_19320 and LIMLP_19325 in L. interrogans serovar Lai belongs to a genomic island (Lai GI B, LA1747-1851) that can excise from the chromosome and form an independent replicative plasmid [26,27].
LIMLP_13165 was among the most down-regulated ORFs when perRB was inactivated. It encodes a secreted protein with sphingomyelinase C and hemolytic activities [28]. Another significantly down-regulated ORF encoded a protein with an acyl CoA acetyl tranferase domain annotated as a putative hemolysin (LIMLP_15470). This ORF is up-regulated when L. interrogans is cultivated in DMC implanted in rats [29].
Among the down-regulated genes, several ORFs encode factors related to oxidative stress. LIMLP_04255 is part of a gene cluster (LIMLP_04240–04285) which code for a putative TonB-dependent transport system repressed by PerRA. We have previously shown that some genes of this cluster (LIMLP_04245, LIMLP_04270 and LIMLP_04280) are involved in L. interrogans tolerance to superoxide [19]. LIMLP_11400 encodes the DNA repair protein RadC and LIMLP_14715 is a homolog of the E. coli Dps, a protein that sequesters iron and protects DNA from oxidative damage. LIMLP_06190 encodes a putative disulfide oxidoreductase with the N-terminal ScdA domain (DUF1858). In S. aureus, ScdA is a di-iron protein involved in repair of oxidatively damaged iron-sulfur cluster proteins [30]. LIMLP_14595 encodes a putative transmembrane lipoprotein with a cytochrome-like domain that shows homology with the CcoP subunit of the cytochrome C oxidase and could function in the respiratory chain or be an enzyme cofactor.
Only 7 out of the 123 differentially-expressed genes in the perRB mutant were also differentially-expressed upon perRA inactivation with a similar inclination (S4 Fig) [19]. LIMLP_02010 and LIMLP_04325 were up-regulated whereas LIMLP_04255, LIMLP_11810, LIMLP_14225, LIMLP_15470 and LIMLP_18235 were down-regulated in the two mutants.
Notably, 82 out of the 123 differentially-expressed ORFs in the perRB mutant were also differentially-expressed upon exposure of L. interrogans to H2O2 (S4 Fig) [19]. Thus, 66% of the PerRB regulon is also regulated by the presence of H2O2. Interestingly, the majority of ORFs down-regulated in the perRB mutant, including the RadC and the Dps-encoding ORFs, were up-regulated in the presence of H2O2 (with Log2FCs of 3.46 and 1.10, respectively) (S4 Fig and Table 1). In contrast, many up-regulated ORFs in the perRB mutant had a lower expression in the presence of H2O2. For instance, the ORFs that code for Cas5 (LIMLP_02880), Cas3 (LIMLP_02885), and two DGCs (LIMLP_05450 and LIMLP_05455) were down-regulated upon exposure to H2O2 with Log2FCs lower than -1.21 (S4 Fig and Table 1).
Concomitant inactivation of perRA and perRB leads to a higher resistance to ROS and to a lower virulence
In order to investigate whether PerRA and PerRB cooperate in regulating the adaptive response to ROS, we inactivated perRA by allelic exchange in the perRB mutant (S5 Fig). This allowed obtaining a double perRAperRB mutant in L. interrogans.
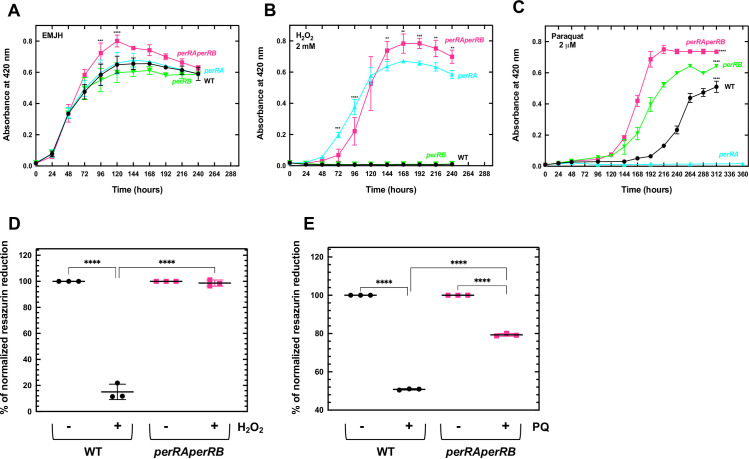
The double perRAperRB mutant had a growth rate comparable to that of the single perRA and perRB mutants and WT strains when L. interrogans were cultivated in EMJH medium (Fig 6A). However, entry in exponential phase was delayed if the culture medium was inoculated with stationary phase-adapted perRAperRB mutant (S5 Fig). We had already shown that a perRA mutant had a higher ability to grow and survive in the presence of deadly concentration of H2O2 but a slower growth in the presence of the superoxide-generating paraquat ([13], and see in Figs S2 and 6B and 6C). In contrast, inactivating perRB led to a higher resistance to paraquat (Figs 5 and 6C). In the presence of H2O2, the double perRAperRB mutant was able to grow with a rate comparable to that of the perRA mutant. In this condition, the perRB mutant and WT strains did not exhibit any growth (Fig 6B). In the presence of paraquat, the double perRAperRB and the perRB mutants entered in logarithmic growth earlier than the WT strain (Fig 6C). Survival tests showed that the perRAperRB mutant had a higher survival than the WT upon exposure to H2O2 (Fig 6D) or to paraquat (Fig 6E). Therefore, the double perRAperRB mutant exhibited cumulative phenotypes of the respective single perRA and perRB mutants when L. interrogans are exposed to ROS.
Fig 6. Effect of concomitant inactivation of perRA and perRB on Leptospira growth in the presence of ROS.
L. interrogans WT (black circles), the single perRA mutant (cyan triangles), the single perRB mutants (green inverted triangles) or the double perRAperRB mutant (pink squares) were cultivated in EMJH medium in the absence (A), or in the presence of 2 mM H2O2 (B) or 2 μM paraquat (C). Growth was assessed by measure of absorbance at 420 nm and the data are means and standard errors of three independent biological replicates. In panel A, statistical significance between the perRAperRB mutant and the other strains was determined by Two-way Anova test (***, p-value<0.0008; ****, p-value<0.0001). In panel B, statistical significance between the perRA and the perRAperRB mutants was determined by two-way Anova test (**, p-value<0.008; ***, p-value<0.0005; ****, p-value<0.0001). In panel C, two-way Anova test indicated that differences between WT, perRB mutant, and perRAperRB mutant were significant from 144 h (****, p-value<0.0001). Quantitative survival tests were performed by incubating exponentially growing WT (black circles) and double perRAperRB mutant (pink squares) with 5 mM H2O2 for 30 min (D) or 100 μM paraquat for 60 min (E). Resazurin reduction was determined as described in the Materials and Methods section. Data are means and standard errors of three independent biological replicates. Statistical significance was determined by a One-way Anova test (****, p-value<0.0001).
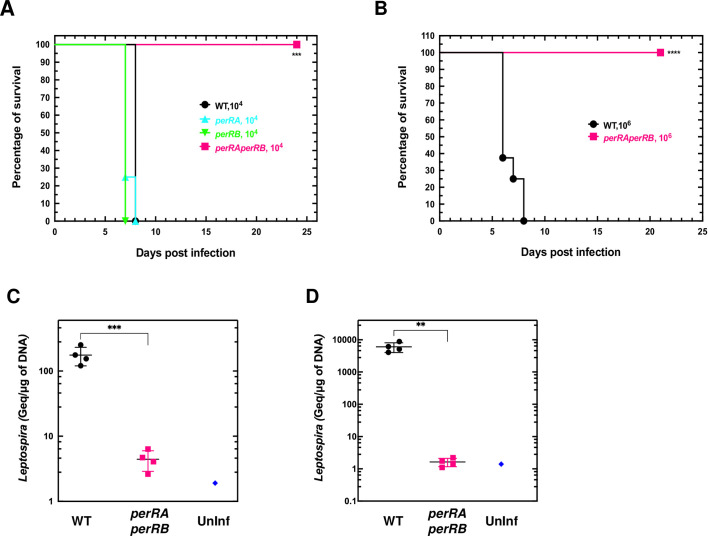
We then tested whether perRA and perRB inactivation had any influence on L. interrogans virulence in the animal model of acute leptospirosis. All hamsters infected intraperitoneally with 104 bacteria of the perRA or perRB single mutant strains exhibited morbidity sign after 7–8 days, similarly to the WT strain (Fig 7A). In contrast, all animals infected intraperitoneally with 104 or 106 bacteria of the double perRAperRB mutant strain did not show any sign of morbidity three weeks post-infection (Fig 7A and 7B). Moreover, the double perRAperRB mutant could not be recovered from kidney and liver of infected animals (Fig 7C and 7D). Therefore, the double perRAperRB mutant exhibited a dramatically reduced virulence and an inability to colonize a host, despite a higher resistance to ROS.
Fig 7. Effect of concomitant inactivation of perRA and perRB on Leptospira virulence.
Virulence (A) was assessed by infecting hamsters (n = 4–8) by peritoneal route with 104 (A) or 106 (B) WT (black circles), 104 single perRA or perRB mutants (cyan triangles and green inverted triangles, respectively), or 104 (A) or 106 (B) double perRAperRB mutant (pink squares) strains as described in Materials and Methods section. Leptospiral load in kidney (C) and liver (D) of hamsters (n = 4) infected with the WT (black circles) or with the perRAperRB mutant (pink squares) was assessed by quantitative PCR as described in the Materials and Methods section. A sample of non-infected hamsters (blue diamonds) was included as a control. Statistical significance in comparison with WT samples was determined by a Log rank Mantel Cox test (in A, *** p-value = 0.0009; in B, **** p-value<0.0001) and by an unpaired t- test (in C, *** p-value = 0.0009; in D, ** p-value = 0.001).
In spite of several attempts, we could not complement the loss of virulence of the double perRAperRB mutant. This prompted us to check the presence of spontaneous mutations in this mutant. Whole DNA sequencing was performed on the WT, perRB and double perRAperRB mutant strains. As seen in S6 Fig, 2 mutations were found in the double perRAperRB mutant that were not present in the perRB mutant, its parental strain. One nucleotide insertion was present in the LIMLP_11570 ORF (encoding a 3-oxoacyl ACP synthase putatively involved in fatty acid synthesis) in the perRAperRB mutant, however a similar insertion was also observed in several L. interrogans isolates from human and animals. One non-synonymous SNP was identified specifically in the double perRAperRB mutant. This SNP was located in the LIMLP_01895 ORF, which encodes a hybrid histidine kinase. This missense mutation led to the conservative substitution of Ala with Val at position 148, between the CheY-like response regulator and PAS domains (see in S6 Fig). The resulting protein still displays intact Asp56 and His294, the two putative phosphorylation sites necessary for signal transduction leading to regulation of effectors.
Concomitant inactivation of perRA and perRB correlates with a pleiotropic effect in L. interrogans gene expression
To further understand the interplay between PerRA and PerRB in controlling the oxidative stress response and virulence in L. interrogans, we performed RNA-Seq experiments on the double perRAperRB mutant and compared its transcriptomic profile with that of WT and single perRA and perRB mutant strains.
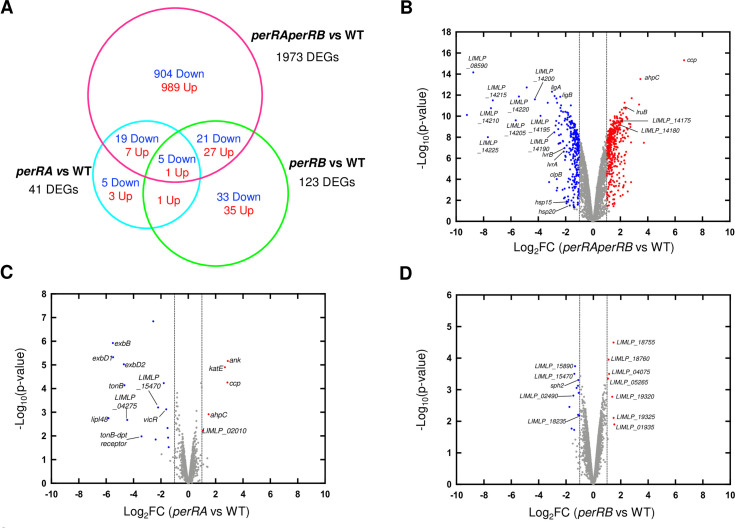
949 and 1024 ORFs were down- and up-regulated, respectively, in the double perRAperRB mutant (Fig 8A and see S3 Table for a complete set of data), which corresponds to almost half of the total coding sequences of L. interrogans. In comparison, only about 1% and 3% of the total coding sequences of L. interrogans were differentially-expressed in the single perRA and perRB mutants, respectively (S2 Table) [19]. Volcano scatter plot representation indicated not only a higher magnitude of fold changes but also a greater statistical significance in the double perRAperRB mutant (Fig 8B and 8D).
Fig 8. Differential gene expression in the perRAperRB mutant.
(A) Venn diagram showing the overlap of differentially-expressed ORFs (with an adjusted p-value < 0.05) in the double perRAperRB mutant (in pink) with those of the perRA mutant (as determined by Zavala-Alvarado et al. [19]) (in cyan) and of the perRB mutant (as determined in this study) (in green). (B)-(D) Volcano scatter representation of differentially-expressed genes in the perRAperRB mutant (B), in the single perRA mutant (as determined by Zavala-Alvarado et al. [19]) (C), and in the single perRB mutant (as determined in this study) (D). Red and blue dots indicate significantly up- and down-regulated genes, respectively, with a Log2FC cutoff of ±1 (dashed vertical lines) and p-value<0.05. Selected genes are labelled.
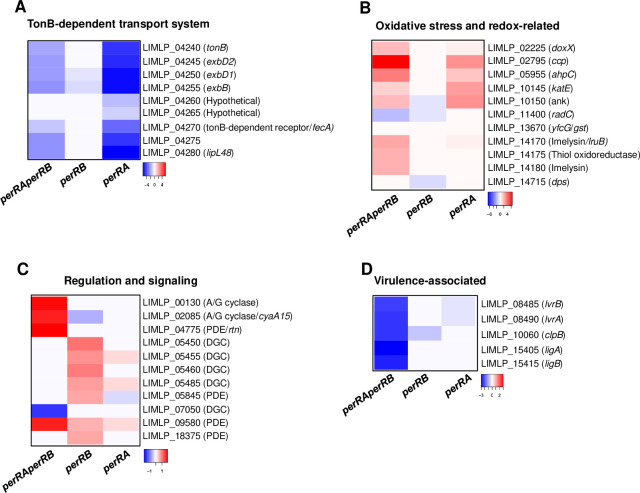
Most of the differentially-expressed ORFs in the perRA mutant were also differentially-expressed in the double perRAperRB mutant (Fig 8A). Many genes of the LIMLP_04240–04285 cluster encoding a putative TonB-dependent transport system, the two-component system VicKR (LIMLP_16720–16725), a putative hemolysin (LIMLP_15470) and several ORFs of unknown function (from LIMLP_14190 to LIMLP_14225) were down-regulated in the perRA [12,19] and perRAperRB mutants (Figs 8 and 9A and S4 Table). Likewise, the ORFs encoding the catalase, AhpC and CCP (LIMLP_10145, LIMLP_05955 and LIMLP_02795, respectively), that are repressed by PerRA and up-regulated in the single perRA mutant [12,19], were also up-regulated in the double perRAperRB mutant (Figs 8 and 9B and S5 Table).
Fig 9. Comparison of differential gene expression in the double perRAperRB mutant with that in the single perRA and perRB mutants.
Expression of selected genes of the TonB-dependent transport cluster (A), involved in oxidative stress and redox homeostasis (B), in regulation and signaling (C), and in virulence (D) determined by RNASeq in the double perRAperRB mutant was compared to those in the single perRA mutant determined by Zavala-Alvarado et al. [19] and single perRB mutant (as determined in this study). Differential expression in each mutant strain was normalized with that in the WT strain. Gene names are indicated on the right. The Heat Map color from blue to red indicates low to high Log2FC.
21 and 27 ORFs that are respectively down- and up-regulated in the perRB mutant were also down- and up-regulated in the perRAperRB mutant (Fig 8A). LIMLP_11400 (encoding RadC), LIMLP_04255, encoding ExbB of the TonB-dependent transport system, the hemolysin-encoding ORF LIMLP_15470, and LIMLP_15890 were down-regulated in the single perRB and double perRAperRB mutants (Fig 8 and S2 and S4 Tables).
Interestingly, the vast majority of the differentially-expressed ORFs in the double perRAperRB mutant did not exhibit any change in their expression in the single perRA and perRB mutants. For instance, among the DGCs and PDEs that were up-regulated in the perRB mutant, only LIMLP_09580 was up-regulated in the double perRAperRB mutant (S2 and S4 and S5 Tables). In fact, the perRAperRB double mutant exhibited a distinct expression pattern of genes involved in signaling (Fig 9C). The LIMLP_07050 ORF that codes for a DGC was down-regulated; two ORFs encoding adenylate/guanylate cyclases (LIMLP_00130 and LIMLP_02085) and the PDE-encoding LIMLP_04775 ORF were up-regulated in the perRAperRB mutant. Finally, only 6 ORFs were differentially expressed in all mutants (Fig 8A), including LIMLP_04255 and LIMLP_15470 (S4 and S5 Tables). Moreover, a substantial number of regulatory factors (transcriptional regulators, two-component systems, sigma factors) were differentially-expressed exclusively in the perRAperRB mutant (S4 and S5 Tables).
In the double perRAperRB mutant, several ORFs encoding factors putatively involved in cell growth (cell division, respiration and cell wall homeostasis), chemotaxis and motility are significantly up-regulated, with a Log2FC greater than 1.5 (S5 Table).
In addition to the PerRA-repressed peroxidases (catalase, AhpC, CCP), other oxidative-stress related factors exhibited a higher expression in the perRAperRB mutant (Fig 9B). DoxX-encoding ORF, which is up-regulated upon concomitant perRA and perRB inactivation (Log2FC 1.65), is an integral membrane protein that interacts with SodA in M. tuberculosis and participates in tolerance to oxidative and redox stress [31]. Two imelysins (LIMLP_14170/LruB and LIML_14180) and a thiol peroxidase (LIML_14175) exhibited also a higher expression in the perRAperRB mutant (Log2FC of 2.36, 1.93, and 2.04 respectively, Fig 9B). All these up-regulated factors (except DoxX) were also up-regulated upon exposure to deadly H2O2 dose [19] and they probably participate in the higher tolerance of the double mutant in the presence of oxidants. Despite the up-regulation of several factors involved in the defense against ROS, the DNA repair protein RadC (encoded by LIMLP_11400) and the glutathione S transferase (encoded by LIMLP_13670) were notably down-regulated in the perRAperRB mutant (Log2FC of -1.9 and -2.3, respectively) (Fig 9B and S4 Table).
Strikingly, several down-regulated ORFs in the double perRAperRB mutant such as clpB, ligA, ligB, and the operon lvrAB have been associated with Leptospira virulence. As in many bacteria, leptospiral ClpB ATPase is involved in disaggregating protein aggregates arising upon stress-induced protein denaturation [32]. ClpB expression is increased upon exposure to H2O2 and it is required for Leptospira virulence [19,33]. The ClpB-encoding ORF (LIMLP_10060) is dramatically down-regulated in the perRAperRB mutant (Log2FC of -2.99) (Figs 8B and 9D and S4 Table).
Another virulence factors in Leptospira are the immunoglobulin-like LigA (LIMLP_15405) and LigB (LIMLP_15415) proteins. These surface-exposed proteins are involved in adhesion to host cells through ECM binding [34] and participate in the immune evasion through binding to the host complement Factor H and C4b binding protein [35]. Simultaneous down-regulation of ligA and ligB expression led to attenuation of Leptospira virulence [36]. LigA and ligB were down-regulated in the perRAperRB mutant (Log2FC of -3 and -2.44, respectively) (Figs 8B and 9D and S4 Table).
LvrA (LIMLP_08490) and lvrB (LIMLP_08485) encode a hybrid histidine kinase and a hybrid response regulator, respectively. Inactivation of the lvrAB operon led to virulence attenuation in L. interrogans [37]. LvrA and lvrB had both a decreased expression in the perRAperRB mutant (Log2FC of -2.3) (Figs 8B and 9D and S4 Table).
Additional ORFs encoding chaperones (the small heat shock proteins Hsp15 and Hsp20) or enzymes involved in protein folding (the disulfide isomerase DsbD and the peptidyl-prolyl cis-trans isomerase SlyD) and degradation (HtpX) were down-regulated in the perRAperRB mutant. DsbD and these two small Hsps were up-regulated in L. interrogans upon exposure to H2O2 [19]. All these factors might protect Leptospira proteostasis under adverse conditions as encountered during infection inside a host.
The down-regulation of the virulence-associated genes together with the differential expression of several other genes was confirmed by RT-qPCR (S7 Fig).
Taken together, these findings indicate that the concomitant inactivation of perRA and perRB correlated with global changes in gene expression, leading to the deregulation of several virulence-associated genes.
Identification of differentially-expressed non-coding RNAs in the perRB and perRAperRB mutants
Intergenic regions were also analyzed to identify differentially expressed predicted non-coding RNAs (ncRNAs). As observed for coding sequences, inactivation of perRB led to the deregulation of only a few putative ncRNAs and most of the changes in expression were below two folds (see S6 Table for a complete set of data). Nonetheless, a few numbers of ncRNAs were significantly down-regulated with a Log2FC below -1 (S7 Table). Some of the differentially-expressed ncRNAs (LepncRNA36, LepncRNA87, LepncRNA89, LepncRNA109, LepncRNA139) were located in the proximate vicinity of differentially-expressed ORFs in the perRB mutant. Three ncRNAs (LepncRNA35, LepncRNA89 and LepncRNA109) were also differentially expressed upon perRA inactivation (S7 Table) [19].
55 putative ncRNAs were differentially-expressed (with a Log2FC cutoff of ±1) in the perRAperRB mutant and several of them were adjacent or overlapped differentially-expressed ORFs (S8 Table). Only a few of these differentially-expressed ncRNAs had an altered expression in the single perRA and perRB mutant (S8 Table) [19].
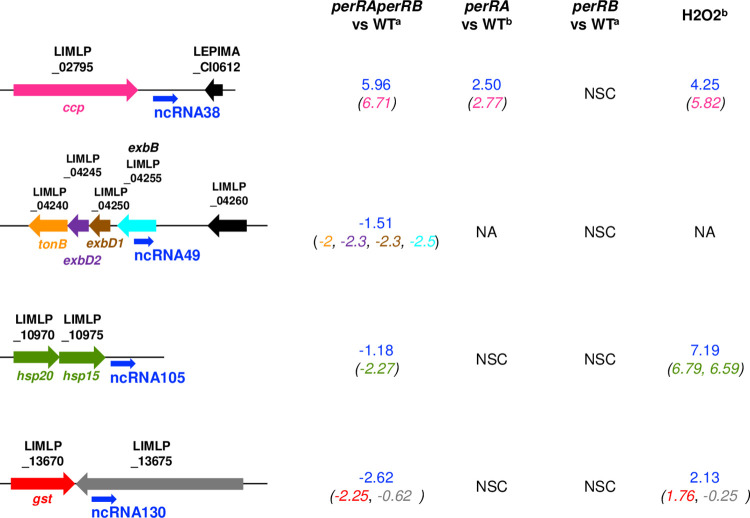
Among the most highly differentially-expressed ncRNAs was LepncRNA38 that was located downstream ccp, a highly up-regulated ORF in the perRAperRB mutant (Fig 10 and S8 Table). LepncRNA38 and ccp were also up-regulated in the perRA mutant [19]. The ncRNA LepncRNA49, which was down-regulated in the perRAperRB mutant, overlapped with exbB (LIMLP_04255), an ORF that was also down-regulated in the double perRAperRB mutant as well as in the single perRA and perRB mutants (Fig 10). The down-regulated LepncRNA105 and LepncRNA130 ncRNAs were located downstream the hsp20-15 operon and gst, respectively, three ORFs whose expression is decreased is the perRAperRB mutant (Fig 10 and S8 Table). It is worth noting that LepncRNA38, LepncRNA105 and LepncRNA130 are up-regulated by H2O2 as were ccp, hsp20-15 and gst ([19]; Fig 10).
Fig 10. Non-coding RNAs expression in the double perRAperRB mutant.
Differential expression of selected ncRNAs (LepncRNA38, 49, 105, and 130) in the perRAperRB mutant (determined in this study) (a) was compared to those in the single perRA mutant, as determined by Zavala-Alvarado et al. [19] (b), in the single perRB mutant (determined by this study) (a), and upon exposure to 1 mM H2O2 for 1h00 as determined by Zavala-Alvarado et al. [19] (b). The location of the ncRNAs LepncRNA38, 49, 105, and 130 were represented schematically with the adjacent or overlapping ORFs. The values indicate the Log2FC of ncRNAs expression normalized with that in WT. The respective expression of these ORFs (Log2FC) are indicated into parenthesis with the same color as their representation in the cartoon. NSC, non-significantly changed; NA, non-applicable.
Altogether, these findings indicate that the absence of both PerRA and PerRB correlates with major changes in the transcriptional activity of many ncRNAs in L. interrogans, that could consequently alter the expression of many ORFs.
Discussion
Virulence mechanisms are poorly characterized in pathogenic Leptospira. These bacteria possess a high number of genes encoding proteins of unknown function (40% of the genomes) and many of them are pathogen-specific. Pathogenic Leptospira spp. lack many classical virulence factors, such as type III to type VI secretion systems, and it is unclear which factors are important for their pathogenesis. It is therefore generally agreed that these pathogens possess unique virulence factors. Nonetheless, studying heme oxygenase and catalase mutants have shown that, in vivo, iron acquisition and defense against peroxide stress are important virulence-associated mechanisms in L. interrogans [11,38]. Catalase is repressed by PerRA [12,19] and genes encoding factors involved in iron uptake are very likely controlled by regulators of the Fur-family.
In addition to PerRA, pathogenic Leptospira contain three other ORFs annotated as Furs. In the present study, we have characterized the L. interrogans Fur-like regulator encoded by LIMLP_05620 and showed that it exhibits characteristic features of a PerR regulator. We consequently named this ORF perRB. Sequence alignment and phylogenetic analyses revealed that PerRB is the closest relative to the already characterized PerRA, and perhaps more importantly, they both do exhibit the canonical amino acid residues that are the hallmark of a PerR. The H2O2 sensing histidine and aspartate residues are conserved in Leptospira PerRA and PerRB and, interestingly, both genes are H2O2-responsive, albeit with different apparent sensitivity. This is consistent with a mechanism whereby PerRA and PerRB would repress their own transcription and dissociate from their promoter upon oxidation by H2O2, leading to alleviation of repression. Moreover, the higher survival of the perRB mutant in the presence of superoxide suggests a derepression of genes encoding defenses against ROS and therefore the participation of PerRB in controlling the adaptation to oxidative stress. Neither perRA nor perRB expression was up-regulated in iron-limiting condition [12]. Although the putative lipoprotein LIMLP_18755 was significantly up-regulated in the perRB mutant and under iron-limiting condition, there was no strong overlap between PerRB regulon and the transcriptional response to iron-limiting condition [12]. Altogether, these findings suggest that LIMLP_05620 encodes a PerR-like rather than a Fur regulator. However, because iron homeostasis and oxidative stress are intertwined, a certain functional relationship has been observed between PerR and Fur. In several bacteria where PerR and Fur coexist, including B. subtilis and C. jejuni, the PerR regulon overlaps with that of Fur [39,40]. In addition, fur and several Fur-regulated genes are also differentially expressed in the presence of H2O2 [41,42]. In fact, PerR represses fur, whose expression is up-regulated in the presence of H2O2 as a consequence of dissociation of PerR from the fur promoter [43,44]. Metal-catalyzed oxidation of the H2O2 sensing residues will be fundamental in establishing that the PerRB regulator is a bona fide PerR.
The coexistence of several PerR regulators in a bacterium is rare. Three PerR-like regulators have been reported only in Gram-positive bacteria such as B. licheniformis and M. smegmatis. It was shown that the three B. licheniformis PerRs sense hydrogen peroxide by histidine oxidation, although with different sensitivity [45]. In M. smegmatis, three Fur-like paralogs displayed the canonical PerR Asp residue involved in H2O2 sensitivity, exhibited H2O2 sensing by metal-catalyzed histidine oxidation and a higher H2O2 resistance was observed when their genes were inactivated [46].
One important question was to understand the mechanism that had led to the coexistence of PerRA and PerRB exclusively in highly virulent species (P1 clade). Virulent mammalian-adapted strains in the Leptospira genus might have originated from a free-living ancestor inhabiting soils. The phylogenetic analysis presented here indicates that the coexistence of PerRA and PerRB is not due to gene duplication. Indeed, PerRA was already present in the leptospirales ancestor whereas PerRB was probably acquired by the most recent common ancestor of the P1 clade by horizontal transfer from a soil/aquatic bacterium of another phylum (the closest homologues being found in the proteobacteria Silvanigrella aquatica). In this scenario, PerRA would had been lost by the P2 clade intermediate species but maintained together with PerRB by the P1 clade species to establish full virulence.
We had previously shown that when perRA was inactivated, L. interrogans acquired a higher resistance to H2O2 explained by the derepression of katE, ahpC and ccp [12,19]. Here, we have demonstrated that inactivating perRB resulted in a higher survival of L. interrogans in the presence of superoxide but it did not affect the survival of L. interrogans in the presence of H2O2. Therefore, even though perRB is up-regulated upon exposure to H2O2 as perRA, the consequence of perRB inactivation is different than that of perRA, suggesting that PerRA and PerRB have a distinct and non-redundant function in Leptospira adaptation to oxidative stress. The distinct distribution of PerRA and PerRB in the Leptospira genus and differences in their respective regulon support the hypothesis of a non-redundant function in the adaptation to oxidative stress.
Phenotypic studies suggest that PerRB represses (directly or indirectly) genes encoding defenses against superoxide. Pathogenic Leptospira species do not encode any SOD or SOR that could be responsible for detoxification of superoxide whereas saprophyte non-pathogenic Leptospira do have such enzymes. Overall, the differentially-expressed genes upon perRB inactivation are mostly Leptospira-specific and poorly characterized. The highest differentially-expressed ORFs were mainly involved in regulation and cell signaling (transcription and sigma factors, adenylate/diguanylate cyclase, TCSs) and could be involved in regulating the adaptation to various challenging stress encountered within a host. Further studies will be required to determine whether superoxide detoxification in L. interrogans is mediated by enzymatic detoxification or metal-dependent scavenging mechanisms and to clarify the exact role of PerRB in controlling those pathways.
The low number of significantly differentially-expressed genes in the perRB mutant when L. interrogans are cultivated in vitro led us to propose that PerRB exerts its function during oxidative stress or upon host-related conditions. Consistent with this hypothesis is the up-regulation of perRB in the presence of lethal H2O2 dose. It is worth noting that there is, to some extent, an overlap between the PerRB regulon and the differentially-expressed genes upon exposure to lethal H2O2 dose [19]. Moreover, the exclusive presence of PerRB in the pathogenic Leptospira clades strongly suggests that PerRB function is more related to regulating adaptation to infection-related conditions rather than to environmental survival. Consistent with this hypothesis is our observation that the viability of the perRB mutant in spring water was comparable to that of the WT (S8 Fig).
One feature of Leptospira genus is the genetic and functional redundancy where multiple genes commonly encode for a similar function. The development of genetic tools has made random and targeted mutagenesis possible in Leptospira, albeit with a low efficiency in comparison to model bacteria. Due to this limitation, only a few Leptospira virulence factors have been identified. The present study is the first to report the concomitant inactivation of two genes. Obtaining a double perRAperRB mutant gave us the unique opportunity to investigate the functional relationship between two PerR-like regulators in a pathogenic bacterium.
In many pathogens which contain only one PerR paralog, such as N. gonorrhoeae, S. pyogenes, and S. aureus, PerR was shown to be involved in virulence [44,47–50]. The single L. interrogans perRA and perRB mutants still retain full virulence in the model for acute leptospirosis (this study and [12]). Interestingly, virulence attenuation was only observed in the double perRAperRB mutant, suggesting an interplay in controlling (directly or indirectly) L. interrogans virulence-associated genes. We could not successfully complement the double perRAperRB mutant. Nevertheless, whole-genome sequencing identified one non-synonymous SNP present only in the double mutant. This SNP resulted in a conservative alanine to valine substitution (both aliphatic amino acids) in the LIMLP_01895 (encoding a hybrid histidine kinase), that should not trigger a drastic functional change in the protein. Further experiments, including inactivation of the LIMLP_01895-encoded kinase, will be necessary to determine whether it plays any role in Leptospira virulence.
The loss of virulence of the double perRAperRB mutant correlated with a large differential gene and ncRNA expression compared not only with the WT but also with the single mutant strains. In other words, the double perRAperRB displayed differential gene expression that were not observed in the single perRA and perRB mutants. While we cannot fully exclude a participation of the LIMLP_01895-encoded kinase in some of the differential gene expression, this could indicate that a subset of genes and ncRNAs is controlled by both PerRA and PerRB. The absence of one regulator could be compensated by the other and most of the genes and ncRNAs that can be regulated by the two regulators would not be differentially expressed in the single mutants. The few genes (LIMLP_02010, LIMLP_04255, LIMLP_04235, LIMLP_11810, LIMLP_14225, LILP_15470, and LIMLP_18235) and ncRNAs that display differential expression in the single mutants in our transcriptomic studies (this study and [19]) indicate a certain functional redundancy of the two regulators even if the phenotypic analyses of the mutants suggest distinct functions. The change in expression of a few regulators when PerRA and PerRB are both absent could lead to major changes in expression of many ORFs or ncRNAs in cascade. The differential expression of many regulators in the double mutant could also explain that the transcriptional profile of the double mutant is not simply the cumulative gene deregulation of the respective single perRA and perRB mutants.
Despite a higher ability to resist ROS, the double perRAperRB mutant lost its virulence; it could not trigger acute leptospirosis-associated morbidity. This could be obviously explained by a significant lower expression of several virulence-associated factors in the double perRAperRB mutant, such as LigA, LigB, LvrA, LvrB, and ClpB. In addition, other dramatically down-regulated genes encode factors such as small Hsps (Hsp15 and Hsp20) for which a role in bacterial virulence is demonstrated in other bacteria including M. tuberculosis [51,52]. Moreover, several differentially-expressed ORFs of unknown function could also be responsible for the loss of virulence of the double perRAperRB mutant.
In summary, this study has allowed to identify a second PerR-like regulator in pathogenic L. interrogans strains that cooperates with PerRA to control the adaptation to oxidative stress. By concomitantly inactivating perRA and perRB, we have unveiled a complex regulatory network that could reveal a putative functional relationship between PerR regulators and Leptospira virulence, most likely through the regulation of virulence- and pathogenicity-associated factors.
Materials and methods
Ethics statement
The protocol for animal experimentation was reviewed and approved by the Institut Pasteur Ethic Committee for Animal Experimentation (CETEA #2016–0019), the competent authority agreed by the French Ministry of Agriculture, for compliance with the French and European regulations on Animal Welfare and with Public Health Service recommendations.
Bacterial strains and growth condition
L. interrogans serovar Manilae strain L495, perRA (M776), perRB (M1474), and the double perRAperRB mutant strains (see S9 Table for a complete description of the strains used in this study) were grown aerobically at 30°C in Ellinghausen-McCullough-Johnson-Harris medium (EMJH) [53] with shaking at 100 rpm. Leptospira growth was followed by measuring the absorbance at 420 nm. β2163 and Π1 E. coli strains were cultivated at 37°C in Luria-Bertani medium with shaking at 37°C in the presence of 0.3 mM thymidine or diaminopimelic acid (Sigma-Aldrich), respectively. When needed, spectinomycin and kanamycin were added at the respective concentration of 50 and 30 μg/ml.
Determination of bacteria viability
Leptospires survival was determined by incubating exponentially growing L. interrogans cells (≈ 108/ml) in EMJH in the presence or absence of 100 μM paraquat for 60 min or 5 mM H2O2 for 30 min at 30°C. Colony-forming unit quantification was performed by diluting bacteria in EMJH and plating on solid EMJH medium. After one month incubation at 30°C, colonies were counted and percent survival (% of colony forming unit, CFU) was calculated as the ratio of CFU for bacteria incubated in the presence of ROS to that for bacteria incubated in the absence of ROS. For the colorimetric assay, 100 μl of leptospires were incubated with resazurin (Alamar Blue reagent, ThermoScientific) for 48h. Leptospires viability was assessed by their capacity to reduce blue resazurin into pink resorufin as described previously [54].
Concomitant inactivation of perRA (LIMLP_10155) and perRB (LIMLP_05620)
PerRA gene (LIMLP_10155/LIC12034/LA1857) was inactivated in the perRB (LIMLP_05620/LIC11158/LA2887) mutant strain (M1474, perRB::KmR) by introduction of a spectinomycin resistance cassette (S5 Fig). For this, a spectinomycin resistance cassette flanked by 0.8 kb sequences homologous to the sequences flanking perRA was created by gene synthesis (GeneArt, Life Technologies) and cloned into a kanamycin-resistant Escherichia coli vector unable to replicate in Leptospira. The obtained suicide plasmid (pKΔperRA) (S10 Table) was introduced in the perRB mutant strain by electroporation as previously described [55] using a Gene Pulser Xcell (Biorad). Individual spectinomycin-resistant colonies were selected on EMJH plates containing 50 μg/ml spectinomycin and screened by PCR (using the P1 and P2 primer set, see S11 Table) for proper replacement of the perRA coding sequence by the spectinomycin resistance cassette. PerRA inactivation in the double perRAperRB mutant was verified by western blot using an anti-PerRA serum (S5 Fig).
Complementation of the perRB mutant
The perRB mutant (perRB::KmR) complementation was performed by expressing the perRB ORF in the pMaORI replicative vector [56]. The perRB (LIMLP_05620) ORF together with its native promoter region (200 bp upstream region) were amplified from genomic DNA of L. interrogans serovar Manilae strain L495 (using the ComPerR2_5Not and ComPerR2_3Xba primer set, S11 Table) and cloned between the Not1 and Xba1 restriction sites in the pMaORI vector. The absence of mutation in the perRB locus in the obtained plasmid (pNB139) was checked by DNA sequencing and the pNB139 plasmid was introduced in the perRB mutant (M1474) by conjugation using the E. coli β2163 conjugating strain as previously described [57]. Leptospira conjugants were selected on EMJH plates containing 50 μg/ml spectinomycin and resistant colonies were then inoculated into liquid EMJH medium supplemented with spectinomycin for further analysis. The restoration of PerRB production in the complemented perRB mutant was verified by western blot (S3 Fig).
Phylogenetic analyses
The sequences homologous to the LIMLP_10155 (PerRA), LIMLP_05620 (PerRB), LIMLP_18590 and LIMLP_04825 proteins were searched with BLASTP version 2.10.0 among the other Leptospira species (Fig 3 and S1 Table) or among the protein sequences of 11,070 representative genomes (Fig 2), as previously described [58]. In that case, only the sequences with an e-value less than 1e-10 and a percentage of similarity greater than 60% were retained. Sequences with percent identity equal to 100% were clustered by CD-HIT version 4.8.1 and only one sequence was retained. The resulting 1671 sequences were subsequently aligned by MAFFT version 7.471. A phylogenetic tree was finally built with IQ-TREE version 2.1.1 under the best-fit model LG + R10. A second phylogenetic tree was made with a subset of sequences to improve the resolution of the separation between PerRA and PerRB. The same procedure was followed, except that the best-fit model used for phylogenetic reconstruction is LG + R5. Both trees were visualized with FigTree version 1.4.4 (https://github.com/rambaut/figtree).
RNA purification
Virulent L. interrogans serovar Manilae strain L495 and perRB (M1474) mutant strains with less than three in vitro passages were used in this study. Four independent biological replicates of exponentially grown L. interrogans WT, perRB (M1474) and double perRAperRB mutant strains were harvested and resuspended in 1 ml TRIzol (ThermoFisher Scientific) and stored at -80°C. Nucleic Acids were extracted with chloroform and precipitated with isopropanol as described earlier [59]. Contaminating genomic DNA was removed by DNAse treatment using the Turbo DNA-free kit (ThermoFisher Scientific) as described by the manufacturer. The integrity of RNAs (RIN > 8.0) was verified by the Agilent Bioanalyzer RNA NanoChips (Agilent technologies, Wilmington, DE).
RNA Sequencing
rRNA were depleted from 0.5 μg of total RNA using the Ribo-Zero rRNA Removal Kit (Bacteria) from Illumina. Sequencing libraries were constructed using the TruSeq Stranded mRNA Sample preparation kit (20020595) following the manufacturer’s instructions (Illumina). The directional libraries were controlled on Bioanalyzer DNA1000 Chips (Agilent Technologies) and concentrations measured with the Qubit dsDNA HS Assay Kit (ThermoFisher). Sequences of 65 bases were generated on the Illumina Hiseq 2500 sequencer.
Reads were cleaned of adapter sequences and low-quality sequences using cutadapt version 1.11 [60]. Only sequences at least 25 nt in length were considered for further analysis. Bowtie version 1.2.2 [61], with default parameters, was used for alignment on the reference genome (L. interrogans serovar Manilae strain UP-MMC-NIID LP, from MicroScope Platform). Genes were counted using featureCounts version 1.4.6-p3 [62] from Subreads package (parameters: -t gene -g locus_tag -s 1).
Count data were analyzed using R version 3.5.1 [63] and the Bioconductor package DESeq2 version 1.20.0 [64]. The normalization and dispersion estimation were performed with DESeq2 using the default parameters and statistical tests for differential expression were performed applying the independent filtering algorithm. Differential expressions were expressed as logarithm to base 2 of fold change (Log2FC). A generalized linear model including the replicate effect as blocking factor was set in order to test for the differential expression between Leptospira samples. Raw p-values were adjusted for multiple testing according to the Benjamini and Hochberg (BH) procedure [65] and genes with an adjusted p-value lower than 0.05 and a Log2FC higher than 1 or lower than -1 were considered differentially expressed. Heat maps and Volcano plots were generated using the Galaxy platform (https://usegalaxy.eu/).
Quantitative RT-PCR experiments
cDNA synthesis was performed with the cDNA synthesis kit (Biorad) according to the manufacturer’s recommendation. Quantitative PCR was conducted in triplicate with the SsoFast EvaGreen Supermix (Biorad) as previously described [19]. FlaB (LIMLP_09410) was chosen as reference gene.
Non-coding RNA identification
Sequencing data from the L. interrogans WT, perRB (M1474) and double perRAperRB mutant strains were processed with Trimmomatic [66] to remove low-quality bases and adapter contaminations. BWA mem (version 0.7.12) was used to discard the reads matching Leptospira rRNA, tRNA or polyA sequences and to assign the resulting reads to Leptospira replicons. Then Rockhopper [67] was used to re-align reads corresponding to separate replicons and to assemble transcripts models. The output was filtered to retain all transcripts longer than 50 nucleotides not overlapping within 10 nucleotides with NCBI annotated genes on the same orientation, and showing a minimum Rockhopper raw count value of 50 in at least two isolates. This high-quality set of new sRNA was subjected to differential expression analysis with Rockhopper, adopting a Benjamini-Hochberg adjusted P-value threshold of 0.01. For each non-coding RNAs, putative function was identified by BLAST using the Rfam database [68].
Infection experiments
WT and mutant L. interrogans strains were cultivated in EMJH medium until the exponential phase and counted under a dark-field microscope using a Petroff-Hauser cell. 104 or 106 bacteria (in 0.5 ml) were injected intraperitoneally in groups of 4–8 male 4 weeks-old Syrian Golden hamsters (RjHan:AURA, Janvier Labs). Animals were monitored daily and sacrificed by carbon dioxide inhalation when endpoint criteria were met (sign of distress, morbidity).
To assess leptospiral load in tissues, liver and kidneys were sampled from infected hamsters (n = 4) (with 106 leptospires) 6–8 or 21 days post-infection for the WT and the perRAperRB mutant, respectively. Genomic DNA was extracted from about 35 mg of tissue using the Maxwell 16 Tissue DNA purification kit (Promega) and total DNA concentrations were normalized to 20 ng/μl. Leptospiral load was assessed by quantitative PCR using the SsoFast EvaGreen Supermix (Biorad) with lipL32 primers (S11 Table). The PCR reactions were run with the CFX96 Real-Time System (Biorad) using the absolute quantification program as follows: 95°C for 3 min followed by 40 cycles of amplification (95°C for 10 s and 55°C for 30 s). Genome equivalents (Geq) were calculated as previously described [69].
Supporting information
Extended phylogenetic tree showing the separation between PerRA (LIMLP_10155 in red) and PerRB (LIMLP_05620 in blue).
(TIF)
L. interrogans WT (black circles), perRA (cyan triangles) and perRB (green inverted triangles) mutant strains were cultivated in EMJH medium at 30°C in the absence (A) or presence of 2 mM H2O2 (B). Leptospira growth was assessed by absorbance at 420 nm. Data are means and standard errors of three independent biological experiments.
(TIF)
L. interrogans WT, perRB mutant and perRB mutant complemented strains were cultivated in EMJH medium at 30°C until the logarithmic phase and lyzed by sonication in 25 mM Tris pH 7.5, 100 mM KCl, 2 mM EDTA, 5 mM DTT, with a protease inhibitors cocktail (cOmplete Mini EDTA-free, Roche). 10 μg of total lyzates were resolved on a 15% SDS-PAGE and transferred on nitrocellulose membrane. PerRB was detected by immunoblot (A) using a rabbit polyclonal antibody at 1/500 dilution. FcpA production was assessed as a control of equal loading using a rabbit polyclonal antibody at 1/1000 dilution. A goat anti-rabbit IgG secondary antibody coupled to the HRP peroxidase (Sigma) was used at a 1/150000 dilution. Detection was performed by chemiluminescence with the Supersignal West Pico PLUS reagent (ThermoScientific). PerRB content was quantified using ImageJ (Schneider et al., Nat Methods 9, 671–675 (2012) https://doi.org/10.1038/nmeth.2089) and normalized by the quantity in the WT strain (B). Data are means and standard errors of three independent biological experiments. Statistical significance was determined by a One-way Anova test in comparison with WT samples (**, p-value = 0.0018).
(TIF)
(A) Venn diagram showing the overlap of differentially-expressed ORFs (with an adjusted p-value < 0.05) in the perRA and perRB mutants. Differentially-expressed genes in the perRB mutant (as determined in this study) (in green) were compared with those in the perRA mutant as determined previously (Zavala-Alvarado et al., PLOS Pathogens. 2020 Oct 6;16(10):e1008904) (in cyan). The down- and up-regulated ORFs in both mutants were indicated in blue and red, respectively. (B) Comparison of differentially-expressed ORFs (with an adjusted p-value < 0.05) in the perRB mutant and upon L. interrogans exposure to H2O2. Log2FC of differentially-expressed ORFs upon L. interrogans exposure to 1 mM H2O2 (as determined previously (Zavala-Alvarado et al., PLOS Pathogens. 2020 Oct 6;16(10):e1008904) was plotted against the Log2FC of differentially-expressed ORFs upon perRB inactivation. Down- and up-regulated ORFs in the perRB mutant were represented by blue and red symbols, respectively, and the name of selected ORFs was indicated. The dashed lines indicate a Log2FC value of zero. Please note that only differentially-expressed ORFs in both conditions were considered.
(TIF)
(A) Schematic representation of the double perRAperRB mutant construction. PerRA (LIMLP_10155) was inactivated by allelic exchange in the transposon perRB mutant. The kanamycin (Km) and spectinomycin (Spc) resistance cassettes inactivating perRB and perRA, respectively, are indicated. (B) Production of PerRA in the WT, in the single perRA and perRB mutants and in the double perRAperRB mutant strains. L. interrogans strains were cultivated in EMJH medium at 30°C until the logarithmic phase and lyzed by sonication in 25 mM Tris pH 7.5, 100 mM KCl, 2 mM EDTA, 5 mM DTT, with a protease inhibitors cocktail (cOmplete Mini EDTA-free, Roche). 10 μg of total lyzates were resolved on a 15% SDS-PAGE and transferred on nitrocellulose membrane. PerRA was detected by immunoblot using a 1/2000 antibody dilution as described previously (Kebouchi et al., J Biol Chem. 2018;293(2):497–509. doi:10.1074/jbc.M117.804443). (C) Growth of stationary phase-adapted WT and perRAperRB mutant strains. L. interrogans WT (black circles) and perRAperRB mutant (pink squares) were cultivated in EMJH medium at 30°C until late stationary phase (7 days after the entry in the stationary phase) and used to inoculate EMJH medium. Bacteria were then cultivated at 30°C and growth was assessed by absorbance at 420 nm. Data are means and standard errors of three independent biological experiments.
(TIF)
(A) Genomic DNA of perRB mutant and perRAperRB mutant strains was extracted with the Maxwell 16 cell DNA purification kit (Promega) and sequenced by Next-generation sequencing. Sequence Reads were processed by fqCleaner and aligned with the reference sequenced genome of Leptospira interrogans serovar Manilae strain UP-MMC-NIID LP (accession numbers CP011931, CP011932, CP011933; Satou et al., Genome Announc. 2015 Aug 13;3(4):e00882-15. doi: 10.1128/genomeA.00882-15. PMID: 26272567; PMCID: PMC4536678.) using Burrows-Wheeler Alignment tool (BWA mem 0.7.5a) (Li & Durbin, Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324). SNP and Indel calling was done with the Genome Analysis Tool Kit GATK2 following the Broad Institute best practices (McKenna et al., Genome Res. 2010;20(9):1297–1303. doi:10.1101/gr.107524.110). The mutations identified (indicated in red) were further confirmed by sequencing PCR-amplified DNA fragments. 358 L. interrogans genomes of a cgMLST Leptospira isolates database (https://bigsdb.pasteur.fr/leptospira/) (Guglielmini et al., PLoS neglected tropical diseases, (2019) 13(4), e0007374. https://doi.org/10.1371/journal.pntd.0007374) were screened for homolog of the affected ORFs using BLASTN 2.9.0+ (Altschul et al., Nucleic Acids Res. 1997;25(17):3389–3402. doi:10.1093/nar/25.17.3389). Identified mutations were searched by alignment using MAFFT version 7.453 (Katoh & Standley, Molecular Biology and Evolution, Volume 30, Issue 4, April 2013, Pages 772–780, https://doi.org/10.1093/molbev/mst010). a Positions, reference sequences, and annotations were indicated according to the L. interrogans serovar Manilae strain UP-MMC-NIID LP (MicroScope Platform (https://mage.genoscope.cns.fr/microscope/home/index.php). b Isolates Id 38, 747, 806, 816, 974, 986, 1058, 1085. (B) Schematic representation of LIMLP_01895. Schematic representation and domain organization of LIMLP_01895 have been determined by ScanProsite (De Castro et al., Nucleic Acids Res. 2006 Jul 1;34(Web Server issue):W362-5.) Nucleotide and corresponding amino acid sequences were indicated from residues 145 to 151 with the mutated codon and amino acid in red. The substituted nucleotide was underlined.
(TIF)
RNAs were extracted from exponentially-grown L. interrogans strains WT or double perRAperRB mutant (m). Expression of the indicated genes was measured by RT-qPCR using the LIMLP_06735 as reference gene. This gene, which encodes a protein with unknown function, was used as a reference gene since its expression was not changed upon inactivation of perRA and perRB. Gene expression in the perRAperRB mutant was normalized against that in the WT strain. Fold change values are indicated in blue. Statistical significance was determined by a Two-way Anova test in comparison with the WT samples (****, p-value<0.0001; **, p-value = 0.0048).
(TIF)
Exponentially growing WT (black circles), perRA (cyan triangles) and perRB (green squares) mutant strains were centrifugated at 2600 g for 15 min and washed three times and resuspended into filter-sterilized spring water (Volvic). All samples were adjusted to 5x108 leptospires/ml. The samples were incubated at RT in darkness and, at the indicated times, leptospires were counted under a dark-field microscope using a Petroff-Hauser cell (A) and their viability was determined by quantification of resazurin reduction using the AlamarBlue reagent (ThermoScientific) (B). Data are means and standard errors of three independent biological experiments. Statistical significance was determined by a Two-way Anova test in comparison with the WT samples (**, p-value = 0.006; *, p-value = 0.0278).
(TIF)
Percentages of homology of PerRA (LIMLP_10155), PerRB (LIMLP_05620), LIMLP_04825 and LIMLP_18590 of L. interrogans serovar Manilae (strain UP-MMC-NIID LP) with their respective orthologs in Leptospira strains of clades P (pathogens) and S (saprophytes). The green color gradient indicates the degree of homology. Grey indicates the presence of a false positive (a different fur-like regulator with low homology) and black indicates the absence of orthologs.
(XLSX)
Differential gene expressions in WT and perRB mutant (M1474) strains are compared with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(XLSX)
Differential gene expressions in WT and perRAperRB double mutant are compared with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(XLS)
Significantly down-regulated genes in the perRAperRB mutant are indicated with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Significantly up-regulated genes in the perRAperRB mutant are indicated with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Putative non-coding RNAs with differential expression in the perRB (M1474) and perRAperRB double mutant are listed in this Table.
(XLSX)
Significantly differentially-expressed non-coding RNAs in the perRB (M1474) mutant are listed in this Table with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Significantly differentially-expressed non-coding RNAs in the perRAperRB mutant are listed in this Table with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
The different strains used in this study are listed in this Table.
(DOCX)
The different plasmids used in this study are listed in this Table.
(DOCX)
All the primers used in this study are listed in this Table.
(XLSX)
Acknowledgments
The authors would like to thank Melissa Caimano and André Grassmann for fruitful discussions. CZA and SGH were part of the Pasteur—Paris University (PPU) International PhD Program.
Data Availability
RNA Sequencing data have been deposited at the NCBI Gene Expression Omnibus (GEO) under the accession number GSE158051 (http://www.ncbi.nlm.nih.gov/geo).
Funding Statement
CZA has received funding from the European Union's Horizon 2020 research and innovation programme (https://ec.europa.eu/programmes/horizon2020/en) under the Marie Sklodowska-Curie grant agreement No 665807 (https://ec.europa.eu/research/mariecurieactions). CZA was awarded a grant from the Fondation Fondation Etchebès-Fondation France (S-CM 16008) (https://www.fondationdefrance.org/fr/fondation/fondation-etchebes). SGH has received funding from the Institut Pasteur (https://www.pasteur.fr/en). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol. 2015;387:65–97. doi: 10.1007/978-3-662-45059-8_5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costa F, Hagan JE, Calcagno J, Kane M, Torgerson P, Martinez-Silveira MS, et al. Global Morbidity and Mortality of Leptospirosis: A Systematic Review. PLoS Negl Trop Dis. 2015. Sep 17;9(9):e0003898–e0003898. doi: 10.1371/journal.pntd.0003898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pijnacker R, Goris MGA, te Wierik MJM, Broens EM, van der Giessen JWB, de Rosa M, et al. Marked increase in leptospirosis infections in humans and dogs in the Netherlands, 2014. Eurosurveillance [Internet]. 2016;21(17). Available from: https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2016.21.17.30211 [DOI] [PubMed] [Google Scholar]
- 4.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nat Rev Microbiol. 2009. Oct;7(10):736–47. doi: 10.1038/nrmicro2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Picardeau M. Virulence of the zoonotic agent of leptospirosis: still terra incognita? Nat Rev Microbiol. 2017;15(5):297–307. doi: 10.1038/nrmicro.2017.5 [DOI] [PubMed] [Google Scholar]
- 6.Imlay JA. Where in the world do bacteria experience oxidative stress? Environmental Microbiology. 2019. Feb 1;21(2):521–30. doi: 10.1111/1462-2920.14445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Winterbourn CC, Kettle AJ. Redox Reactions and Microbial Killing in the Neutrophil Phagosome. Antioxidants & Redox Signaling. 2012. Aug 13;18(6):642–60. doi: 10.1089/ars.2012.4827 [DOI] [PubMed] [Google Scholar]
- 8.Marangoni A, Accardo S, Aldini R, Guardigli M, Cavrini F, Sambri V, et al. Production of reactive oxygen species and expression of inducible nitric oxide synthase in rat isolated Kupffer cells stimulated by Leptospira interrogans and Borrelia burgdorferi. World J Gastroenterol. 2006. May 21;12(19):3077–81. doi: 10.3748/wjg.v12.i19.3077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Araújo AM, Reis EAG, Athanazio DA, Ribeiro GS, Hagan JE, Araujo GC, et al. Oxidative stress markers correlate with renal dysfunction and thrombocytopenia in severe leptospirosis. Am J Trop Med Hyg. 2014. Apr;90(4):719–23. doi: 10.4269/ajtmh.13-0667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erdogan HM, Karapehlivan M, Citil M, Atakisi O, Uzlu E, Unver A. Serum sialic acid and oxidative stress parameters changes in cattle with leptospirosis. Veterinary Research Communications. 2008. Apr 1;32(4):333–9. doi: 10.1007/s11259-008-9036-z [DOI] [PubMed] [Google Scholar]
- 11.Eshghi A, Lourdault K, Murray GL, Bartpho T, Sermswan RW, Picardeau M, et al. Leptospira interrogans Catalase Is Required for Resistance to H2O2 and for Virulence. Blanke SR, editor. Infect Immun. 2012. Nov 1;80(11):3892. doi: 10.1128/IAI.00466-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lo M, Murray GL, Khoo CA, Haake DA, Zuerner RL, Adler B. Transcriptional response of Leptospira interrogans to iron limitation and characterization of a PerR homolog. Infect Immun. 2010. Nov;78(11):4850–9. doi: 10.1128/IAI.00435-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kebouchi M, Saul F, Taher R, Landier A, Beaudeau B, Dubrac S, et al. Structure and function of the Leptospira interrogans peroxide stress regulator (PerR), an atypical PerR devoid of a structural metal-binding site. J Biol Chem. 2018. Jan 12;293(2):497–509. doi: 10.1074/jbc.M117.804443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbig AF, Helmann JD. Roles of metal ions and hydrogen peroxide in modulating the interaction of the Bacillus subtilis PerR peroxide regulon repressor with operator DNA. Mol Microbiol. 2001. Aug;41(4):849–59. doi: 10.1046/j.1365-2958.2001.02543.x [DOI] [PubMed] [Google Scholar]
- 15.Jacquamet L, Traoré D a. K, Ferrer J-L, Proux O, Testemale D, Hazemann J-L, et al. Structural characterization of the active form of PerR: insights into the metal-induced activation of PerR and Fur proteins for DNA binding. Mol Microbiol. 2009. Jul;73(1):20–31. doi: 10.1111/j.1365-2958.2009.06753.x [DOI] [PubMed] [Google Scholar]
- 16.Traoré DAK, El Ghazouani A, Ilango S, Dupuy J, Jacquamet L, Ferrer J-L, et al. Crystal structure of the apo-PerR-Zn protein from Bacillus subtilis. Mol Microbiol. 2006. Sep;61(5):1211–9. doi: 10.1111/j.1365-2958.2006.05313.x [DOI] [PubMed] [Google Scholar]
- 17.Lee J-W, Helmann JD. The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature. 2006. Mar 16;440(7082):363–7. doi: 10.1038/nature04537 [DOI] [PubMed] [Google Scholar]
- 18.Traoré DAK, El Ghazouani A, Jacquamet L, Borel F, Ferrer J-L, Lascoux D, et al. Structural and functional characterization of 2-oxo-histidine in oxidized PerR protein. Nat Chem Biol. 2009. Jan;5(1):53–9. doi: 10.1038/nchembio.133 [DOI] [PubMed] [Google Scholar]
- 19.Zavala-Alvarado C, Sismeiro O, Legendre R, Varet H, Bussotti G, Bayram J, et al. The transcriptional response of pathogenic Leptospira to peroxide reveals new defenses against infection-related oxidative stress. PLOS Pathogens. 2020. Oct 6;16(10):e1008904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caux-Thang C, Parent A, Sethu R, Maïga A, Blondin G, Latour J-M, et al. Single asparagine to arginine mutation allows PerR to switch from PerR box to fur box. ACS Chem Biol. 2015. Mar 20;10(3):682–6. doi: 10.1021/cb500783g [DOI] [PubMed] [Google Scholar]
- 21.Parent A, Caux-Thang C, Signor L, Clémancey M, Sethu R, Blondin G, et al. Single glutamate to aspartate mutation makes ferric uptake regulator (Fur) as sensitive to H2O2 as peroxide resistance regulator (PerR). Angew Chem Int Ed Engl. 2013. Sep 23;52(39):10339–43. doi: 10.1002/anie.201304021 [DOI] [PubMed] [Google Scholar]
- 22.Satou K, Shimoji M, Tamotsu H, Juan A, Ashimine N, Shinzato M, et al. Complete Genome Sequences of Low-Passage Virulent and High-Passage Avirulent Variants of Pathogenic Leptospira interrogans Serovar Manilae Strain UP-MMC-NIID, Originally Isolated from a Patient with Severe Leptospirosis, Determined Using PacBio Single-Molecule Real-Time Technology. Genome Announc. 2015. Aug 13;3(4):e00882–15. doi: 10.1128/genomeA.00882-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thibeaux R, Soupé-Gilbert M-E, Kainiu M, Girault D, Bierque E, Fernandes J, et al. The zoonotic pathogen Leptospira interrogans mitigates environmental stress through cyclic-di-GMP-controlled biofilm production. npj Biofilms and Microbiomes. 2020. Jun 12;6(1):24. doi: 10.1038/s41522-020-0134-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xiao G, Kong L, Che R, Yi Y, Zhang Q, Yan J, et al. Identification and Characterization of c-di-GMP Metabolic Enzymes of Leptospira interrogans and c-di-GMP Fluctuations After Thermal Shift and Infection. Frontiers in Microbiology. 2018;9:764. doi: 10.3389/fmicb.2018.00764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Domingos RF, Vieira ML, Romero EC, Gonçales AP, de Morais ZM, Vasconcellos SA, et al. “Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions”. BMC Microbiology. 2012. Mar 30;12(1):50. doi: 10.1186/1471-2180-12-50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourhy P, Salaün L, Lajus A, Médigue C, Boursaux-Eude C, Picardeau M. A Genomic Island of the Pathogen Leptospira interrogans Serovar Lai Can Excise from Its Chromosome. Infect Immun. 2007. Feb 1;75(2):677. doi: 10.1128/IAI.01067-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He P, Sheng Y-Y, Shi Y-Z, Jiang X-G, Qin J-H, Zhang Z-M, et al. Genetic diversity among major endemic strains of Leptospira interrogans in China. BMC Genomics. 2007. Jul 1;8:204–204. doi: 10.1186/1471-2164-8-204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Narayanavari SA, Lourdault K, Sritharan M, Haake DA, Matsunaga J. Role of sph2 Gene Regulation in Hemolytic and Sphingomyelinase Activities Produced by Leptospira interrogans. PLOS Neglected Tropical Diseases. 2015. Aug 14;9(8):e0003952. doi: 10.1371/journal.pntd.0003952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caimano MJ, Sivasankaran SK, Allard A, Hurley D, Hokamp K, Grassmann AA, et al. A Model System for Studying the Transcriptomic and Physiological Changes Associated with Mammalian Host-Adaptation by Leptospira interrogans Serovar Copenhageni. PLOS Pathogens. 2014. Mar 13;10(3):e1004004. doi: 10.1371/journal.ppat.1004004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Overton TW, Justino MC, Li Y, Baptista JM, Melo AMP, Cole JA, et al. Widespread Distribution in Pathogenic Bacteria of Di-Iron Proteins That Repair Oxidative and Nitrosative Damage to Iron-Sulfur Centers. J Bacteriol. 2008. Mar 15;190(6):2004. doi: 10.1128/JB.01733-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nambi S, Long JE, Mishra BB, Baker R, Murphy KC, Olive AJ, et al. The Oxidative Stress Network of Mycobacterium tuberculosis Reveals Coordination between Radical Detoxification Systems. Cell Host & Microbe. 2015. Jun 10;17(6):829–37. doi: 10.1016/j.chom.2015.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krajewska J, Modrak-Wójcik A, Arent ZJ, Więckowski D, Zolkiewski M, Bzowska A, et al. Characterization of the molecular chaperone ClpB from the pathogenic spirochaete Leptospira interrogans. PLOS ONE. 2017. Jul 10;12(7):e0181118. doi: 10.1371/journal.pone.0181118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lourdault K, Cerqueira GM, Wunder EA, Picardeau M. Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect Immun. 2011. Sep;79(9):3711–7. doi: 10.1128/IAI.05168-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choy HA, Kelley MM, Chen TL, Møller AK, Matsunaga J, Haake DA. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infect Immun. 2007/02/12 ed. 2007. May;75(5):2441–50. doi: 10.1128/IAI.01635-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castiblanco-Valencia MM, Fraga TR, Silva LB da, Monaris D, Abreu PAE, Strobel, et al. Leptospiral Immunoglobulin-like Proteins Interact With Human Complement Regulators Factor H, FHL-1, FHR-1, and C4BP. The Journal of Infectious Diseases. 2012. Mar 15;205(6):995–1004. doi: 10.1093/infdis/jir875 [DOI] [PubMed] [Google Scholar]
- 36.Pappas CJ, Picardeau M. Control of Gene Expression in Leptospira spp. by Transcription Activator-Like Effectors Demonstrates a Potential Role for LigA and LigB in Leptospira interrogans Virulence. Appl Environ Microbiol. 2015/09/04 ed. 2015. Nov;81(22):7888–92. doi: 10.1128/AEM.02202-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Adhikarla H, Wunder EA Jr, Mechaly AE, Mehta S, Wang Z, Santos L, et al. Lvr, a Signaling System That Controls Global Gene Regulation and Virulence in Pathogenic Leptospira. Front Cell Infect Microbiol. 2018. Feb 23;8:45–45. doi: 10.3389/fcimb.2018.00045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murray GL, Srikram A, Henry R, Puapairoj A, Sermswan RW, Adler B. Leptospira interrogans requires heme oxygenase for disease pathogenesis. Microbes and Infection. 2009. Feb 1;11(2):311–4. doi: 10.1016/j.micinf.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 39.Hayashi K, Ohsawa T, Kobayashi K, Ogasawara N, Ogura M. The H2O2 Stress-Responsive Regulator PerR Positively Regulates srfA Expression in Bacillus subtilis. Journal of Bacteriology. 2005;187(19):6659–67. doi: 10.1128/JB.187.19.6659-6667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palyada K, Sun Y-Q, Flint A, Butcher J, Naikare H, Stintzi A. Characterization of the oxidative stress stimulon and PerR regulon of Campylobacter jejuni. BMC Genomics. 2009. Oct 18;10(1):481. doi: 10.1186/1471-2164-10-481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mostertz J, Scharf C, Hecker M, Homuth G. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Vol. 150, Microbiology. Microbiology Society; 2004. p. 497–512. doi: 10.1099/mic.0.26665-0 [DOI] [PubMed] [Google Scholar]
- 42.Zhou A, He Z, Redding-Johanson AM, Mukhopadhyay A, Hemme CL, Joachimiak MP, et al. Hydrogen peroxide-induced oxidative stress responses in Desulfovibrio vulgaris Hildenborough. Environmental Microbiology. 2010;12(10):2645–57. doi: 10.1111/j.1462-2920.2010.02234.x [DOI] [PubMed] [Google Scholar]
- 43.Fuangthong M, Herbig AF, Bsat N, Helmann JD. Regulation of the Bacillus subtilis fur and perR Genes by PerR: Not All Members of the PerR Regulon Are Peroxide Inducible. Journal of Bacteriology. 2002;184(12):3276–86. doi: 10.1128/JB.184.12.3276-3286.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horsburgh MJ, Ingham E, Foster SJ. In Staphylococcus aureus, Fur Is an Interactive Regulator with PerR, Contributes to Virulence, and Is Necessary for Oxidative Stress Resistance through Positive Regulation of Catalase and Iron Homeostasis. Journal of Bacteriology. 2001;183(2):468–75. doi: 10.1128/JB.183.2.468-475.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim J-H, Ji C-J, Ju S-Y, Yang Y-M, Ryu S-H, Kwon Y, et al. Bacillus licheniformis Contains Two More PerR-Like Proteins in Addition to PerR, Fur, and Zur Orthologues. PLOS ONE. 2016. May 13;11(5):e0155539. doi: 10.1371/journal.pone.0155539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee H-N, Ji C-J, Lee H-H, Park J, Seo Y-S, Lee J-W, et al. Roles of three FurA paralogs in the regulation of genes pertaining to peroxide defense in Mycobacterium smegmatis mc2155. Molecular Microbiology. 2018;108(6):661–82. doi: 10.1111/mmi.13956 [DOI] [PubMed] [Google Scholar]
- 47.Brenot A, King KY, Caparon MG. The PerR regulon in peroxide resistance and virulence of Streptococcus pyogenes. Molecular Microbiology. 2005;55(1):221–34. doi: 10.1111/j.1365-2958.2004.04370.x [DOI] [PubMed] [Google Scholar]
- 48.Rea RB, Gahan CGM, Hill C. Disruption of putative regulatory loci in Listeria monocytogenes demonstrates a significant role for Fur and PerR in virulence. Infect Immun. 2004. Feb;72(2):717–27. doi: 10.1128/IAI.72.2.717-727.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ricci S, Janulczyk R, Björck L. The Regulator PerR Is Involved in Oxidative Stress Response and Iron Homeostasis and Is Necessary for Full Virulence of Streptococcus pyogenes. Infect Immun. 2002. Sep 1;70(9):4968. doi: 10.1128/IAI.70.9.4968-4976.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu H-J, Seib KL, Srikhanta YN, Kidd SP, Edwards JL, Maguire TL, et al. PerR controls Mn-dependent resistance to oxidative stress in Neisseria gonorrhoeae. Molecular Microbiology. 2006;60(2):401–16. doi: 10.1111/j.1365-2958.2006.05079.x [DOI] [PubMed] [Google Scholar]
- 51.Yuan Y, Crane DD, Simpson RM, Zhu Y, Hickey MJ, Sherman DR, et al. The 16-kDa α-crystallin (Acr) protein of Mycobacterium tuberculosis is required for growth in macrophages. Proc Natl Acad Sci USA. 1998. Aug 4;95(16):9578. doi: 10.1073/pnas.95.16.9578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stewart GR, Newton SM, Wilkinson KA, Humphreys IR, Murphy HN, Robertson BD, et al. The stress-responsive chaperone α-crystallin 2 is required for pathogenesis of Mycobacterium tuberculosis. Molecular Microbiology. 2005;55(4):1127–37. doi: 10.1111/j.1365-2958.2004.04450.x [DOI] [PubMed] [Google Scholar]
- 53.Ellinghausen HC, Mccullough WG. Nutrition of Leptospira pomona and growth of 13 other serotypes: a serum-free medium employing oleic albumin complex. Am J Vet Res. 1965. Jan;26:39–44. [PubMed] [Google Scholar]
- 54.Mouville C, Benaroudj N. Survival Tests for Leptospira spp. Methods Mol Biol. 2020;2134:215–28. doi: 10.1007/978-1-0716-0459-5_20 [DOI] [PubMed] [Google Scholar]
- 55.Picardeau M, Brenot A, Saint Girons I. First evidence for gene replacement in Leptospira spp. Inactivation of L. biflexa flaB results in non-motile mutants deficient in endoflagella. Molecular Microbiology. 2001. Apr 1;40(1):189–99. doi: 10.1046/j.1365-2958.2001.02374.x [DOI] [PubMed] [Google Scholar]
- 56.Pappas CJ, Benaroudj N, Picardeau M. A Replicative Plasmid Vector Allows Efficient Complementation of Pathogenic Leptospira Strains. Parales RE, editor. Appl Environ Microbiol. 2015. May 1;81(9):3176. doi: 10.1128/AEM.00173-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Picardeau M. Conjugative transfer between Escherichia coli and Leptospira spp. as a new genetic tool. Appl Environ Microbiol. 2007/11/09 ed. 2008 Jan;74(1):319–22. doi: 10.1128/AEM.02172-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vincent AT, Schiettekatte O, Goarant C, Neela VK, Bernet E, Thibeaux R, et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl Trop Dis. 2019. May 23;13(5):e0007270–e0007270. doi: 10.1371/journal.pntd.0007270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zavala-Alvarado C, Benaroudj N. The Single-Step Method of RNA Purification Applied to Leptospira. Methods Mol Biol. 2020;2134:41–51. doi: 10.1007/978-1-0716-0459-5_5 [DOI] [PubMed] [Google Scholar]
- 60.Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet.journal; Vol 17, No 1: Next Generation Sequencing Data AnalysisDO—1014806/ej171200 [Internet]. 2011. May 2; Available from: http://journal.embnet.org/index.php/embnetjournal/article/view/200 [Google Scholar]
- 61.Langmead B, Trapnell C, Pop M, Salzberg SL. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009. Mar 4;10(3):R25. doi: 10.1186/gb-2009-10-3-r25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2013. Nov 13;30(7):923–30. doi: 10.1093/bioinformatics/btt656 [DOI] [PubMed] [Google Scholar]
- 63.R core Team. R, a language and environment for statistcial computing [Internet]. 2016. Available from: https://www.gbif.org/en/tool/81287/r-a-language-and-environment-for-statistical-computing [Google Scholar]
- 64.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biology. 2014. Dec 5;15(12):550. doi: 10.1186/s13059-014-0550-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological). 1995;57(1):289–300. [Google Scholar]
- 66.Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014. Aug 1;30(15):2114–20. doi: 10.1093/bioinformatics/btu170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McClure R, Balasubramanian D, Sun Y, Bobrovskyy M, Sumby P, Genco CA, et al. Computational analysis of bacterial RNA-Seq data. Nucleic Acids Res. 2013. Aug;41(14):e140–e140. doi: 10.1093/nar/gkt444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kalvari I, Argasinska J, Quinones-Olvera N, Nawrocki EP, Rivas E, Eddy SR, et al. Rfam 13.0: shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Research. 2017. Nov 3;46(D1):D335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Evangelista KV, Lourdault K. Evaluation of Vaccine Candidates against Leptospirosis using Golden Syrian Hamsters. In: Koizumi N, Picardeau M, editors. Leptospira spp: Methods and Protocols [Internet]. New York, NY: Springer US; 2020. p. 257–70. Available from: 10.1007/978-1-0716-0459-5_23 [DOI] [PubMed] [Google Scholar]
- 70.Madeira F, Park Y mi, Lee J, Buso N, Gur T, Madhusoodanan N, et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Research. 2019. Jul 2;47(W1):W636–41. doi: 10.1093/nar/gkz268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelley LA, Mezulis S, Yates CM, Wass MN, Sternberg MJE. The Phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols. 2015. Jun 1;10(6):845–58. doi: 10.1038/nprot.2015.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Extended phylogenetic tree showing the separation between PerRA (LIMLP_10155 in red) and PerRB (LIMLP_05620 in blue).
(TIF)
L. interrogans WT (black circles), perRA (cyan triangles) and perRB (green inverted triangles) mutant strains were cultivated in EMJH medium at 30°C in the absence (A) or presence of 2 mM H2O2 (B). Leptospira growth was assessed by absorbance at 420 nm. Data are means and standard errors of three independent biological experiments.
(TIF)
L. interrogans WT, perRB mutant and perRB mutant complemented strains were cultivated in EMJH medium at 30°C until the logarithmic phase and lyzed by sonication in 25 mM Tris pH 7.5, 100 mM KCl, 2 mM EDTA, 5 mM DTT, with a protease inhibitors cocktail (cOmplete Mini EDTA-free, Roche). 10 μg of total lyzates were resolved on a 15% SDS-PAGE and transferred on nitrocellulose membrane. PerRB was detected by immunoblot (A) using a rabbit polyclonal antibody at 1/500 dilution. FcpA production was assessed as a control of equal loading using a rabbit polyclonal antibody at 1/1000 dilution. A goat anti-rabbit IgG secondary antibody coupled to the HRP peroxidase (Sigma) was used at a 1/150000 dilution. Detection was performed by chemiluminescence with the Supersignal West Pico PLUS reagent (ThermoScientific). PerRB content was quantified using ImageJ (Schneider et al., Nat Methods 9, 671–675 (2012) https://doi.org/10.1038/nmeth.2089) and normalized by the quantity in the WT strain (B). Data are means and standard errors of three independent biological experiments. Statistical significance was determined by a One-way Anova test in comparison with WT samples (**, p-value = 0.0018).
(TIF)
(A) Venn diagram showing the overlap of differentially-expressed ORFs (with an adjusted p-value < 0.05) in the perRA and perRB mutants. Differentially-expressed genes in the perRB mutant (as determined in this study) (in green) were compared with those in the perRA mutant as determined previously (Zavala-Alvarado et al., PLOS Pathogens. 2020 Oct 6;16(10):e1008904) (in cyan). The down- and up-regulated ORFs in both mutants were indicated in blue and red, respectively. (B) Comparison of differentially-expressed ORFs (with an adjusted p-value < 0.05) in the perRB mutant and upon L. interrogans exposure to H2O2. Log2FC of differentially-expressed ORFs upon L. interrogans exposure to 1 mM H2O2 (as determined previously (Zavala-Alvarado et al., PLOS Pathogens. 2020 Oct 6;16(10):e1008904) was plotted against the Log2FC of differentially-expressed ORFs upon perRB inactivation. Down- and up-regulated ORFs in the perRB mutant were represented by blue and red symbols, respectively, and the name of selected ORFs was indicated. The dashed lines indicate a Log2FC value of zero. Please note that only differentially-expressed ORFs in both conditions were considered.
(TIF)
(A) Schematic representation of the double perRAperRB mutant construction. PerRA (LIMLP_10155) was inactivated by allelic exchange in the transposon perRB mutant. The kanamycin (Km) and spectinomycin (Spc) resistance cassettes inactivating perRB and perRA, respectively, are indicated. (B) Production of PerRA in the WT, in the single perRA and perRB mutants and in the double perRAperRB mutant strains. L. interrogans strains were cultivated in EMJH medium at 30°C until the logarithmic phase and lyzed by sonication in 25 mM Tris pH 7.5, 100 mM KCl, 2 mM EDTA, 5 mM DTT, with a protease inhibitors cocktail (cOmplete Mini EDTA-free, Roche). 10 μg of total lyzates were resolved on a 15% SDS-PAGE and transferred on nitrocellulose membrane. PerRA was detected by immunoblot using a 1/2000 antibody dilution as described previously (Kebouchi et al., J Biol Chem. 2018;293(2):497–509. doi:10.1074/jbc.M117.804443). (C) Growth of stationary phase-adapted WT and perRAperRB mutant strains. L. interrogans WT (black circles) and perRAperRB mutant (pink squares) were cultivated in EMJH medium at 30°C until late stationary phase (7 days after the entry in the stationary phase) and used to inoculate EMJH medium. Bacteria were then cultivated at 30°C and growth was assessed by absorbance at 420 nm. Data are means and standard errors of three independent biological experiments.
(TIF)
(A) Genomic DNA of perRB mutant and perRAperRB mutant strains was extracted with the Maxwell 16 cell DNA purification kit (Promega) and sequenced by Next-generation sequencing. Sequence Reads were processed by fqCleaner and aligned with the reference sequenced genome of Leptospira interrogans serovar Manilae strain UP-MMC-NIID LP (accession numbers CP011931, CP011932, CP011933; Satou et al., Genome Announc. 2015 Aug 13;3(4):e00882-15. doi: 10.1128/genomeA.00882-15. PMID: 26272567; PMCID: PMC4536678.) using Burrows-Wheeler Alignment tool (BWA mem 0.7.5a) (Li & Durbin, Bioinformatics. 2009;25(14):1754–1760. doi:10.1093/bioinformatics/btp324). SNP and Indel calling was done with the Genome Analysis Tool Kit GATK2 following the Broad Institute best practices (McKenna et al., Genome Res. 2010;20(9):1297–1303. doi:10.1101/gr.107524.110). The mutations identified (indicated in red) were further confirmed by sequencing PCR-amplified DNA fragments. 358 L. interrogans genomes of a cgMLST Leptospira isolates database (https://bigsdb.pasteur.fr/leptospira/) (Guglielmini et al., PLoS neglected tropical diseases, (2019) 13(4), e0007374. https://doi.org/10.1371/journal.pntd.0007374) were screened for homolog of the affected ORFs using BLASTN 2.9.0+ (Altschul et al., Nucleic Acids Res. 1997;25(17):3389–3402. doi:10.1093/nar/25.17.3389). Identified mutations were searched by alignment using MAFFT version 7.453 (Katoh & Standley, Molecular Biology and Evolution, Volume 30, Issue 4, April 2013, Pages 772–780, https://doi.org/10.1093/molbev/mst010). a Positions, reference sequences, and annotations were indicated according to the L. interrogans serovar Manilae strain UP-MMC-NIID LP (MicroScope Platform (https://mage.genoscope.cns.fr/microscope/home/index.php). b Isolates Id 38, 747, 806, 816, 974, 986, 1058, 1085. (B) Schematic representation of LIMLP_01895. Schematic representation and domain organization of LIMLP_01895 have been determined by ScanProsite (De Castro et al., Nucleic Acids Res. 2006 Jul 1;34(Web Server issue):W362-5.) Nucleotide and corresponding amino acid sequences were indicated from residues 145 to 151 with the mutated codon and amino acid in red. The substituted nucleotide was underlined.
(TIF)
RNAs were extracted from exponentially-grown L. interrogans strains WT or double perRAperRB mutant (m). Expression of the indicated genes was measured by RT-qPCR using the LIMLP_06735 as reference gene. This gene, which encodes a protein with unknown function, was used as a reference gene since its expression was not changed upon inactivation of perRA and perRB. Gene expression in the perRAperRB mutant was normalized against that in the WT strain. Fold change values are indicated in blue. Statistical significance was determined by a Two-way Anova test in comparison with the WT samples (****, p-value<0.0001; **, p-value = 0.0048).
(TIF)
Exponentially growing WT (black circles), perRA (cyan triangles) and perRB (green squares) mutant strains were centrifugated at 2600 g for 15 min and washed three times and resuspended into filter-sterilized spring water (Volvic). All samples were adjusted to 5x108 leptospires/ml. The samples were incubated at RT in darkness and, at the indicated times, leptospires were counted under a dark-field microscope using a Petroff-Hauser cell (A) and their viability was determined by quantification of resazurin reduction using the AlamarBlue reagent (ThermoScientific) (B). Data are means and standard errors of three independent biological experiments. Statistical significance was determined by a Two-way Anova test in comparison with the WT samples (**, p-value = 0.006; *, p-value = 0.0278).
(TIF)
Percentages of homology of PerRA (LIMLP_10155), PerRB (LIMLP_05620), LIMLP_04825 and LIMLP_18590 of L. interrogans serovar Manilae (strain UP-MMC-NIID LP) with their respective orthologs in Leptospira strains of clades P (pathogens) and S (saprophytes). The green color gradient indicates the degree of homology. Grey indicates the presence of a false positive (a different fur-like regulator with low homology) and black indicates the absence of orthologs.
(XLSX)
Differential gene expressions in WT and perRB mutant (M1474) strains are compared with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(XLSX)
Differential gene expressions in WT and perRAperRB double mutant are compared with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(XLS)
Significantly down-regulated genes in the perRAperRB mutant are indicated with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Significantly up-regulated genes in the perRAperRB mutant are indicated with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Putative non-coding RNAs with differential expression in the perRB (M1474) and perRAperRB double mutant are listed in this Table.
(XLSX)
Significantly differentially-expressed non-coding RNAs in the perRB (M1474) mutant are listed in this Table with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
Significantly differentially-expressed non-coding RNAs in the perRAperRB mutant are listed in this Table with gene numbering according to Leptospira interrogans serovar Manilae strain UP-MMC-NIID-LP genome.
(DOCX)
The different strains used in this study are listed in this Table.
(DOCX)
The different plasmids used in this study are listed in this Table.
(DOCX)
All the primers used in this study are listed in this Table.
(XLSX)
Data Availability Statement
RNA Sequencing data have been deposited at the NCBI Gene Expression Omnibus (GEO) under the accession number GSE158051 (http://www.ncbi.nlm.nih.gov/geo).