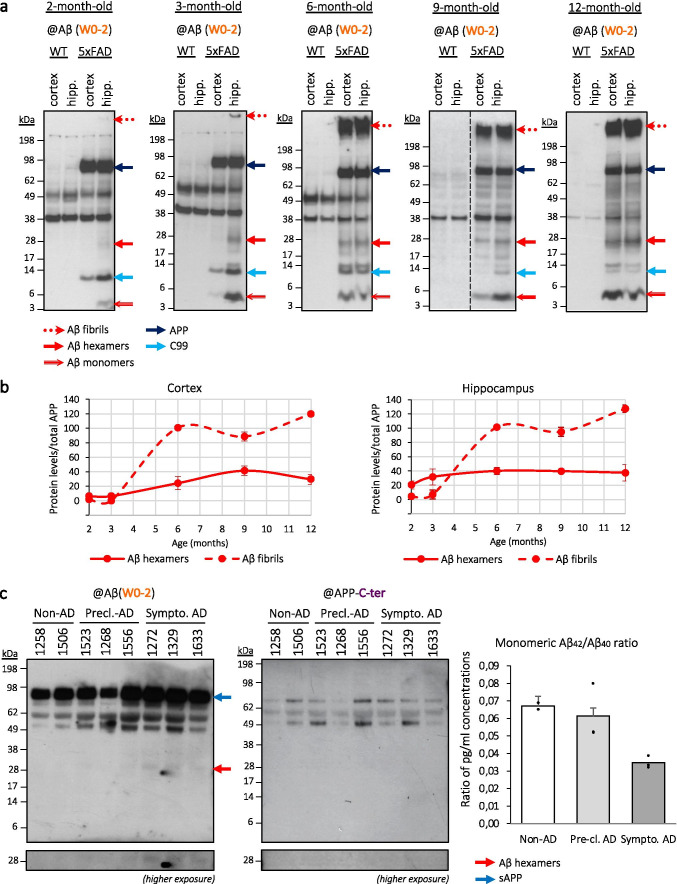
Fig. 5.
Identification of hexameric-like Aβ assemblies in the context of AD. a Detection of Aβ assemblies in brain samples from an amyloid mouse model (5xFAD). Cortices and hippocampi of euthanized mice were lysed and analyzed by Western blotting with the anti-Aβ (W0-2) antibody. Aβ fibrils appear stuck in the wells and hexameric-like Aβ assemblies are detected at ~ 28 kDa. To note, C99 fragments (~ 10 kDa) and Aβ monomers (~ 4.5 kDa) are also detected in all 5xFAD samples and reflect an efficient metabolism of the human APP protein expressed in these mice. Dashed lines indicate that proteins were run on the same gel, but lanes are not contiguous. Hipp. hippocampus. b The signal intensities of Aβ hexamers and Aβ fibrils were quantified relatively to the APP signal. Samples used for quantitative analysis derived from the same experiment, with Western blots processed in parallel. The displayed graphs represent the profile of Aβ assemblies as related to both the analyzed brain area (cortex, hippocampus) and the age (2, 3, 6, 9, 12 months of age) (min N = 3 each). c Identification of ~ 28 kDa, hexameric-like, Aβ assemblies in the cerebrospinal fluid (CSF) of AD patients. Western blotting analysis was performed using the anti-Aβ (W0-2) and anti-APP-C-ter antibodies. Higher exposures were performed for a better appreciation of the bands of interest; full images can be found in Supplementary Fig.S5. Dosage of monomeric Aβ42/Aβ40 by ECLIA immunoassay confirmed the correct classification of individuals, with a reduction in ratio along with AD progression. sAPP soluble APP, Pre-cl. Pre-clinical, Sympto. symptomatic