Extended Data Fig. 3. Controls for transsynaptic tracing experiments.
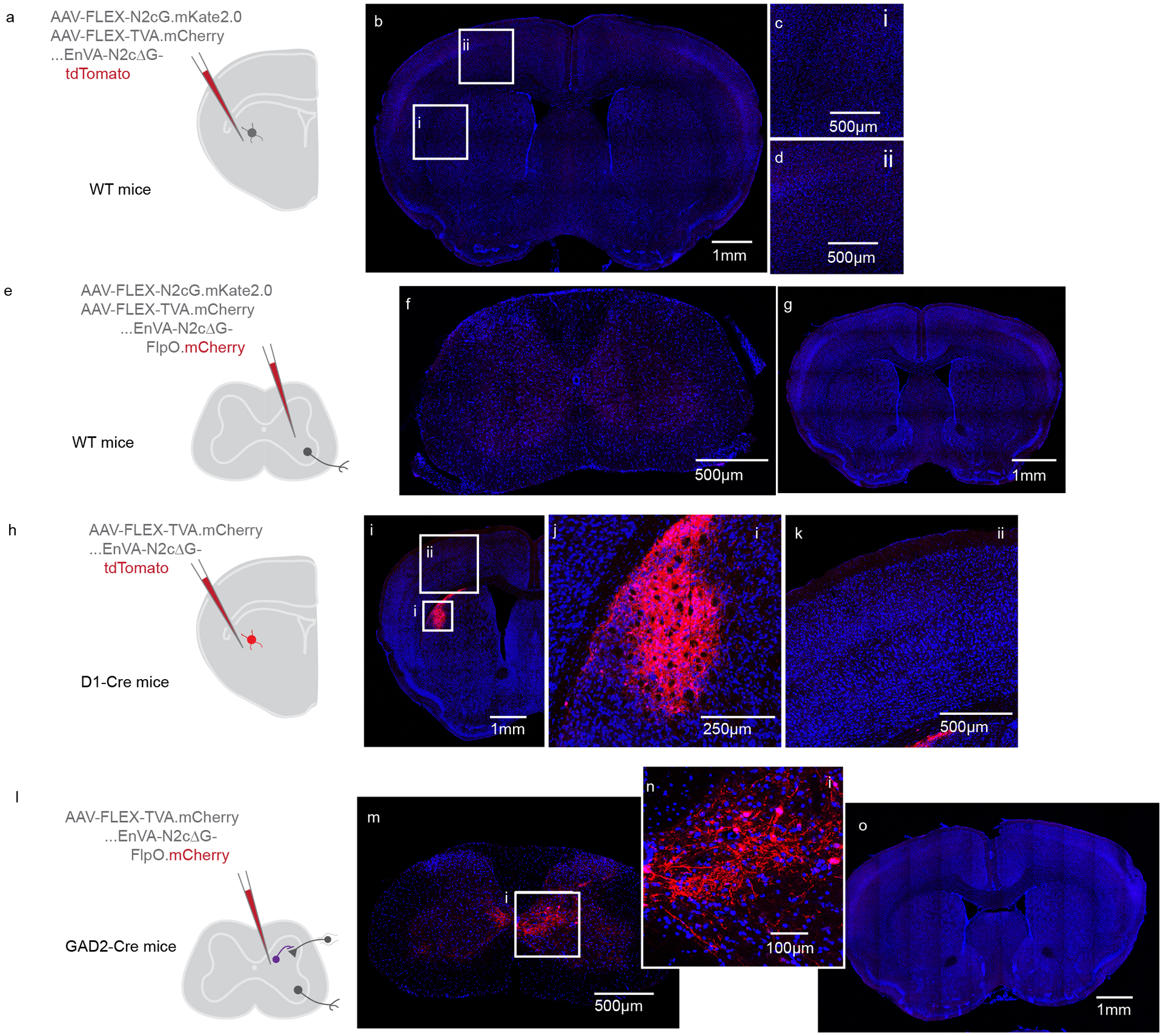
(A) Experimental strategy to confirm Cre-dependent expression of AAVs-FLEX encoding rabies glycoprotein and TVA.mCherry in DLS, as well as dependence of EnVA-N2cΔG-tdTomato infection on expression of TVA. The AAVs were injected into wild type mice, followed by injection of rabies. (B-D) Photomicrographs illustrating the absence of any mCherry or tdTomato labeling in the brain. Representative of N=3. (E) Experimental strategy to confirm Cre-dependent expression of AAVs-FLEX encoding rabies glycoprotein and TVA.mCherry in the spinal cord, as well as dependence of EnVA-N2cΔG-FlpO.mCherry infection on expression of TVA. (F-G) Photomicrographs illustrating the absence of any mCherry or tdTomato labeling in the spinal cord or brain. Representative of N=3. (H) Experimental strategy to confirm dependency of transsynaptic spread on rabies glycoprotein, in the DLS. AAV-FLEX-TVA.mCherry was injected into DLS of D1-Cre mice. AAV-FLEX-N2cG was omitted from the injection. (I-K) Injecting EnVA-N2cΔG-tdTomato led to local tdTomato expression, but no expression in presynaptic inputs to DLS. Representative of N=1. (L) Experimental strategy to confirm dependency of transsynaptic spread on rabies glycoprotein, in the spinal cord. AAV-FLEX-TVA.mCherry was injected into spinal cord of GAD2-Cre mice. (M-O) Injecting EnVA-N2cΔG-FlpO.mCherry led to local mCherry expression, but no expression in presynaptic inputs to spinal cord. Representative of N=3.
