Extended Data Figure 1 |. Structural comparison of human and invertebrate ALK.
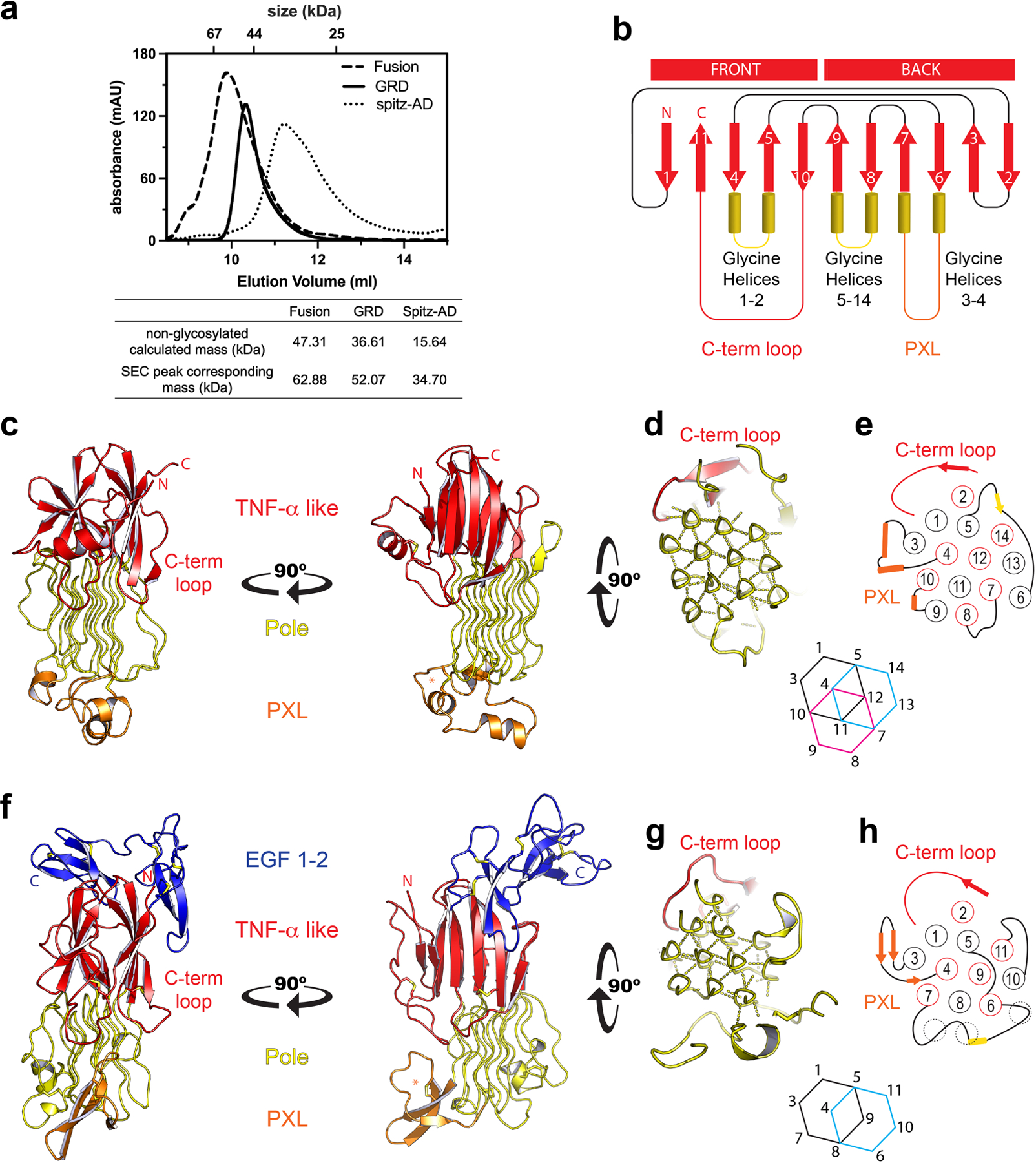
a, Size exclusion chromatography of each protein was carried out using a Superdex 75 Increase 10/300 GL column. The non-glycosylated mass was calculated from the protein sequence. The mass corresponding to each SEC peak was determined based on the log (MW) versus elution volume plot of standards for the column. The SEC peak mass of each protein is consistent with a glycosylated monomer. b, ALK’s glycine helices project downward from the TNF-α like region. Unique to ALK, strand 10 crosses over strands 4 and 5 to terminate the fold, producing a surface terminal loop, “C-term loop”. The GRD has a distinct topology, and does not form the jelly-roll characteristic of TNF-α domains c, The GRD of human ALK. d, The hexagonal array of the Pole, and e, the order and topology of the Pole (red circles into the page, black out of the page). f, C. elegans ALK adopts a similar overall architecture to the human GRD. However, the invertebrate PXL is structurally different, forming a β-hairpin rather than helices (orange). g, The Pole of invertebrate ALK is also smaller, with 11 glycine helices that form 2 complete hexagons. h, The helical strands are in the same order and topology as in human ALK. Interestingly, the missing glycine helices of the invertebrate Pole are partially matched by non PG-II loops that interact with the hexagonal array (dashed circles). The invertebrate GRD structure additionally includes a C-terminal cysteine-rich region that leads up to the transmembrane domain. This region has 10 cysteines and forms 2 EGF-like domains (f, blue).
