Abstract
Fracture induces systemic bone loss in mice and humans, and a first (index) fracture increases the risk of future fracture at any skeletal site more in men than women. The etiology of this sex difference is unknown, but fracture may induces a greater systemic bone loss response in males. Also sex differences in systemic muscle loss after fracture have not been examined. We investigated sex differences in systemic bone and muscle loss after transverse femur fracture in 3-month-old male and female C57BL/6J mice. Whole-body and regional bone mineral content and density (BMC and BMD), trabecular and cortical bone microstructure, muscle contractile force, muscle mass, and muscle fiber size were quantified at multiple time points post-fracture. Serum concentrations of inflammatory cytokines (IL-1β, IL-6, and TNF-α) were measured 1-day post-fracture. One day post-fracture, IL-6 and Il-1B were elevated in fracture mice of both sexes, but TNF-α was only elevated in male fracture mice. Fracture reduced BMC, BMD, and trabecular bone microstructural properties in both sexes 2 weeks post-fracture, but declines were greater in males. Muscle contractile force, mass, and fiber size decreased primarily in the fractured limb at 2 weeks post-fracture and females showed a trend towards greater muscle loss. Bone and muscle properties recovered by 6 weeks post-fracture. Overall, post-fracture systemic bone loss is greater in males, which may contribute to sex differences in subsequent fracture risk. In both sexes, muscle loss is primarily confined to the injured limb and fracture may induce greater inflammation in males.
Keywords: fracture, systemic bone loss, muscle, sex
Introduction
Osteoporotic fracture is a significant health concern for older individuals of both sexes, with annual medical costs estimated at 4.25 billion.1–4 In the first 1–2 years following a first (index) fracture, the risk of a subsequent fracture increases 2–10 fold.5,6 While reduced physical function and other cofactors may contribute, another explanation is that fracture induces a period of systemic bone loss that increases risk of a subsequent fracture. Consistent with this hypothesis, our previous study showed that in elderly Caucasian women, an incident fracture is associated with a period of accelerated decline in hip bone mineral density (BMD).7 Importantly, index fracture increases future fracture risk more in males than females.8–10 While behavior and lifestyle may partly explain sex differences, males may also experience greater systemic bone loss after fracture than women.
Our lab recently demonstrated that both young (3 month-old) and middle-aged (12 month-old) female mice lost bone at distant sites two weeks after femur fracture, and this was associated with increased osteoclast number and systemic inflammation, as well as decreases in voluntary movement three days after fracture.11 Although the mechanisms of post-fracture systemic bone loss have not been well-studied, fracture releases pro-inflammatory cytokines (e.g. IL-1β, IL-6, and TNF-α), which increase osteoclast differentiation and activity and inhibit osteoblast bone formation.12–14 Chronic inflammation in auto-immune diseases such as rheumatoid arthritis leads to bone loss and osteoporosis.15,16 Although it is currently unclear if the acute period of inflammation after fracture is similarly able to induce significant bone resorption, injury elicits greater circulating levels of pro-inflammatory cytokines in males and ovariectomized females than intact females. 17–20 Conversely, females may exhibit higher concentrations of anti-inflammatory cytokines.21,22 Therefore differences in inflammation may contribute to sex differences in post-traumatic bone loss.
Bone fracture also causes disuse atrophy and damage-induced degeneration of the overlying skeletal muscle, which may increase risk of sarcopenia and fall induced fracture.23–25 It has been hypothesized that males are potentially more sensitive to inflammation-mediated muscle loss, but disuse-induced atrophy appears greater in females.26 Thus, it is unclear if fracture induces systemic muscle loss and if the magnitude differs between sexes.
The current study investigates sex-differences in systemic bone and muscle loss as well as inflammation after femur fracture. We hypothesize that 2 weeks after fracture, male mice will exhibit greater bone loss, greater reductions in mechanical properties, and a greater decrease in muscle strength and fiber size than female mice, and that males will recover less bone by 6 weeks post-fracture. We specifically evaluate changes in bone microarchitecture at 2 weeks post-fracture, because our prior work showed systemic bone loss reached a maximum at this time point in young (3 month old) female mice before recovering by 6 weeks after fracture. Two weeks post-fracture is also a significant time point from the perspective of fracture healing. Callus volume and mineralized callus volume reach a maxima, which may correlate to a peak in the systemic response to fracture.27 Whole body as well as lumber spine and femoral diaphysis bone mineral density was quantified at 1,2, 4, and 6 weeks to allow more fine-grained evaluation of the time course and magnitude of bone loss. We further hypothesize that concentrations of circulating pro-inflammatory cytokines will also be higher in males than females 1-day post-fracture.
Materials and Methods
Animals
One hundred and twenty C57BL/6J mice (60 female and 60 male) were obtained from the Jackson Laboratory (Bar Harbor, ME) at 10 weeks of age and acclimated for 2 weeks prior to the start of experimental procedures. Mice were cared for in accordance with the guidelines set by the National Institutes of Health (NIH) on the care and use of laboratory animals. Mice had ad libitum access to food (Harlan irradiated 2918 chow) and autoclaved water and were monitored by husbandry staff at least once a day. All procedures were approved by the Institutional Animal Care and Use Committee of the University of California, Davis. Animal numbers for study groups are reported in Table 1.
Table 1.
Sample Size by Time Point, Sex, and Fracture Location
| Fracture Group | Day 1 | Week 2 | Week 6 | |||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Right Leg Fracture | 9 | 10 | 5 | 4 | 5 | 5 |
| Left Leg Fracture | 0 | 0 | 4 | 4 | 4 | 3 |
| Control | 10 | 10 | 10 | 9 | 10 | 9 |
| Euthanized | 1 | 0 | 1 | 3 | 1 | 3 |
Male and female mice were randomly assigned to fracture and control groups. Groups of mice (6–10 animals per sex per experimental group) were euthanized 1 day after fracture to assess inflammation and at 2 and 6 weeks after fracture to measure bone microstructural and mechanical properties, as well as muscle mass and fiber size. All mice were euthanized via CO2 asphyxiation followed by cervical dislocation.
Creation of Femur Fracture
Mice received buprenorphine (0.05 mg/kg subcutaneous injection) pre-operation and were anesthetized via isoflurane (2–3% concentration) inhalation. Hair was removed from the anterior leg and thigh with a depilatory. Femur fractures were induced as originally described by Bonnarens and Einhorn28. As described below, the equipment for measuring muscle contractile force could only be used on the right leg. Therefore, to allow for evaluation of contractile force in both the contralateral (uninjured) and ipsilateral (injured) leg, fracture was created in either the right or left leg for the 2- and 6-week time points (Females: 10 right femur fracture, 10 left femur fractures; Males: 9 right femur fracture, 13 left femur fracture) (Table 1). For the Day 1 time point, fractures were created only on the right side since muscle contractile force was not measured in these animals. During fracture surgery, an incision was made on the side of the knee and a 0.254 mm stainless steel wire pin inserted into the medullary canal to stabilize the fracture. Transverse fractures were then created with a controlled impact using a fracture apparatus, in which the dropped wedge struck the lateral aspect of the femur diaphysis, creating a transverse fracture in the mid-diaphyseal region. Control mice received anesthesia and analgesia only, with no surgical procedures. Immediately post-fracture, mice were imaged with planar radiographs (HE100/30þX-ray machine, MinXRay, Northbrook IL, USA) with a CXD1–31 plate (Canon, LakeSuccess, NY, USA) to confirm pin positioning and a transverse mid-diaphyseal fracture. Nine mice were euthanized due to poor pin placement or poor fracture location (Table 1). Full weight-bearing and unrestricted activity was permitted postoperatively. All mice received an additional dose of buprenorphine (0.05 mg/kg) 8 hours post-operation, and then twice daily for two days thereafter. Mice did not receive antibiotics during post-op care, but were visually inspected daily for signs of infection, which were not observed in any mice.
Cabinet Dual-Energy X-Ray Absorptiometry (DXA)
At Baseline (Day 0) and 1, 2, 4, and 6 weeks after fracture, mice were anesthetized via isoflurane inhalation and imaged using a cabinet x-ray system (Mozart, Kubtec Medical Imaging, Stratford CT). Whole-body Bone Mineral Content (BMC) and Bone Mineral Density (BMD) were measured using the manufacturer’s analysis software, which automatically excluded the head and intermedullary pin (Fig. 1A). We also measured BMC and BMD of the lumbar spine and contralateral femoral diaphysis. The Region of Interest (ROI) for the lumbar spine was defined from the cranial border of L4 to the caudal end of L6 (Fig. 2A). The femoral diaphysis ROI was manually selected to encompass the middle third of the femur (Fig. 2F).
Figure 1: Dual-energy X-ray absorptiometry (DXA) imaging of whole-body BMD and BMC.
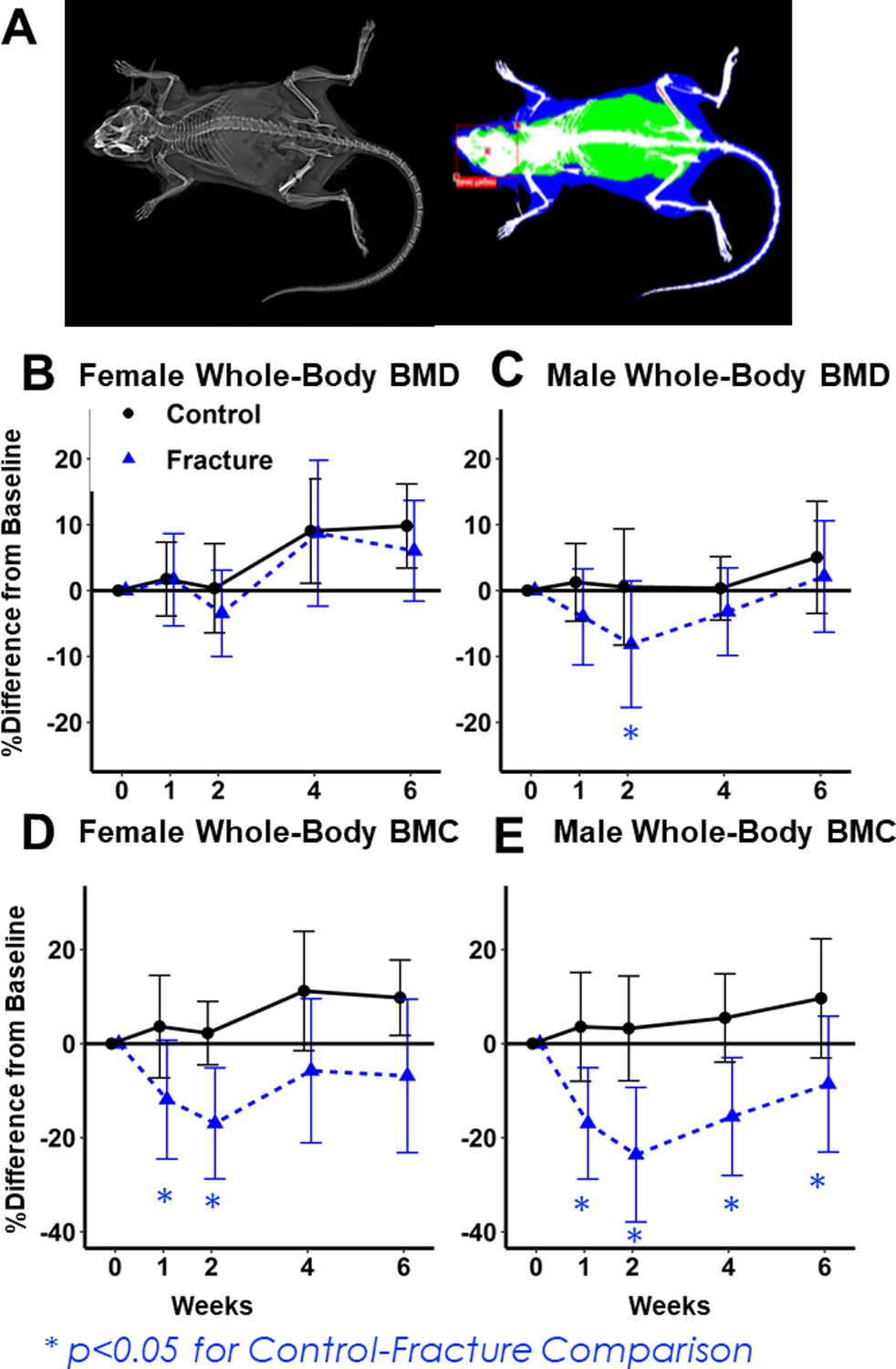
(A) Representative image for DXA analysis. (B, C) Percent change from baseline in Females (B) and Males (C) BMD. Decreases in Fracture mice relative to Controls were greater in Males. (D,E) Percent change from baseline in Female (D) and Male (E) BMC. Percent decreases were greatest in Male Fracture mice.
Figure 2: Dual-energy X-ray absorptiometry (DXA) imaging of lumbar spine and uninjured (contralateral) femur diaphysis BMD and BMC.
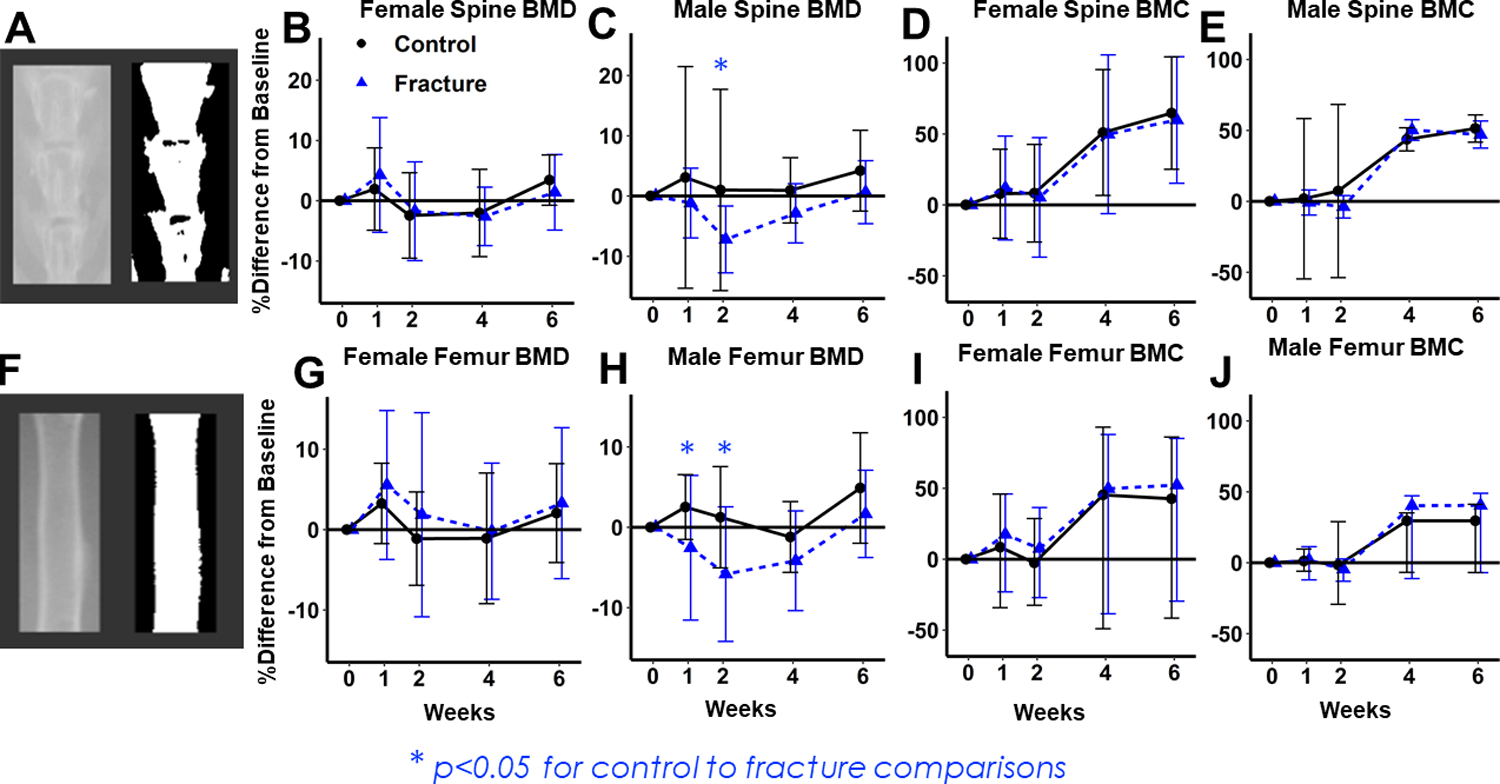
(A) Representative image of lumbar spine region of interest. (B,C) Percent change in BMD from baseline in Females (B) and Males (C) Percent decrease in Fracture group is greater in males. (D,E) Percent change from baseline in BMC for Females (D) and Males (E), with no significant differences between Fracture and Control Mice. (F) Representative image of uninjured femur diaphysis region of interest. (G,H) Percent change in BMD from baseline in Females (G) and Males (H) Percent decrease in Fracture group is greater in Males. (I,J) Percent change from baseline in BMC for Females (I) and Males (J), with no significant differences between Fracture and Control Mice.
Trabecular and Cortical Bone Microstructural Properties
After euthanasia, L5 vertebrae and contralateral femora were preserved in 70% ethanol. Bones were scanned using micro-computed tomography (SCANCO Medical, μCT 35, Brüttisellen, Switzerland) with 6 mm nominal voxel size (X-ray tube potential= 55 kVp, current= 114mA, integration time= 900 ms, number of projections= 1000/180°).29 All analyses were performed using the manufacturer’s analysis software. Trabecular bone was analyzed at the L5 vertebral body and distal femoral metaphysis. Trabecular ROIs were manually drawn on transverse images excluding the cortical surface. The ROI for the L5 vertebral bodies extended from the cranial growth plate to the caudal growth plate, excluding transverse processes. The ROI for the distal femoral metaphysis extended 250 slices proximally from the metaphyseal growth plate (1500 µm). Analysis of cortical bone in the femoral diaphysis was performed by contouring transverse slices, with a 1200 μm (200 slices) ROI centered on the midpoint of the femur. Trabecular bone volume fraction (BV/TV), trabecular thickness (Tb.Th), trabecular number (Tb.N), and other microstructural parameters were determined using the manufacturer’s analysis software.
3-Point Mechanical Testing of Femora
3-point bending was used to determine structural and material properties of contralateral (uninjured) femora using a materials testing system (ELF 3200, TA Instruments, New Castle, DE, USA). Femora were rehydrated for 10–15 minutes in PBS solution. The span length of the lower supports was 8 mm, and the femur was positioned to load the anterior aspect of the bone in tension. A 1–2 N preload was applied to ensure contact with the upper platen, then loading was applied at a displacement rate of 0.01 mm/sec until failure. Resulting force and displacement data were recorded at 50 Hz and analyzed to determine whole bone stiffness, yield force, ultimate force, energy to fracture, and post-yield displacement. Material properties of the femur diaphysis were calculated using structural measurements of the femoral midshaft determined with μCT using previously established beam theory equations.30
Gastrocnemius Tetanic Force Measurement
Maximum muscle contraction force of the gastrocnemius muscle of the fractured and contralateral limbs was assessed at Baseline (day 0) and Weeks 1, 2, 4, and 6 post-fracture for all mice. Hair was removed from hindlimbs using a depilatory cream. Mice were anesthetized using isoflurane inhalation, and gastrocnemius muscle tetanic force was measured on the right leg with a 305C-LR Dual Mode Muscle Lever and Footplate system (Aurora Scientific, Aurora, Ontario) (Fig. 5A). Briefly, the right mouse hindlimb ankle, knee, and hip joints were positioned at 90-degree angles in the system. A pin was then tightened posterior to the knee joint to stabilize the limb, and the foot was firmly fastened to a foot plate with athletic tape. Two electrodes were cleaned and inserted into the right gastrocnemius muscle. The maximum contractile force was reported following stimulation at 125 Hz.
Figure 5: In-vivo testing of gastrocnemius tetanic force.
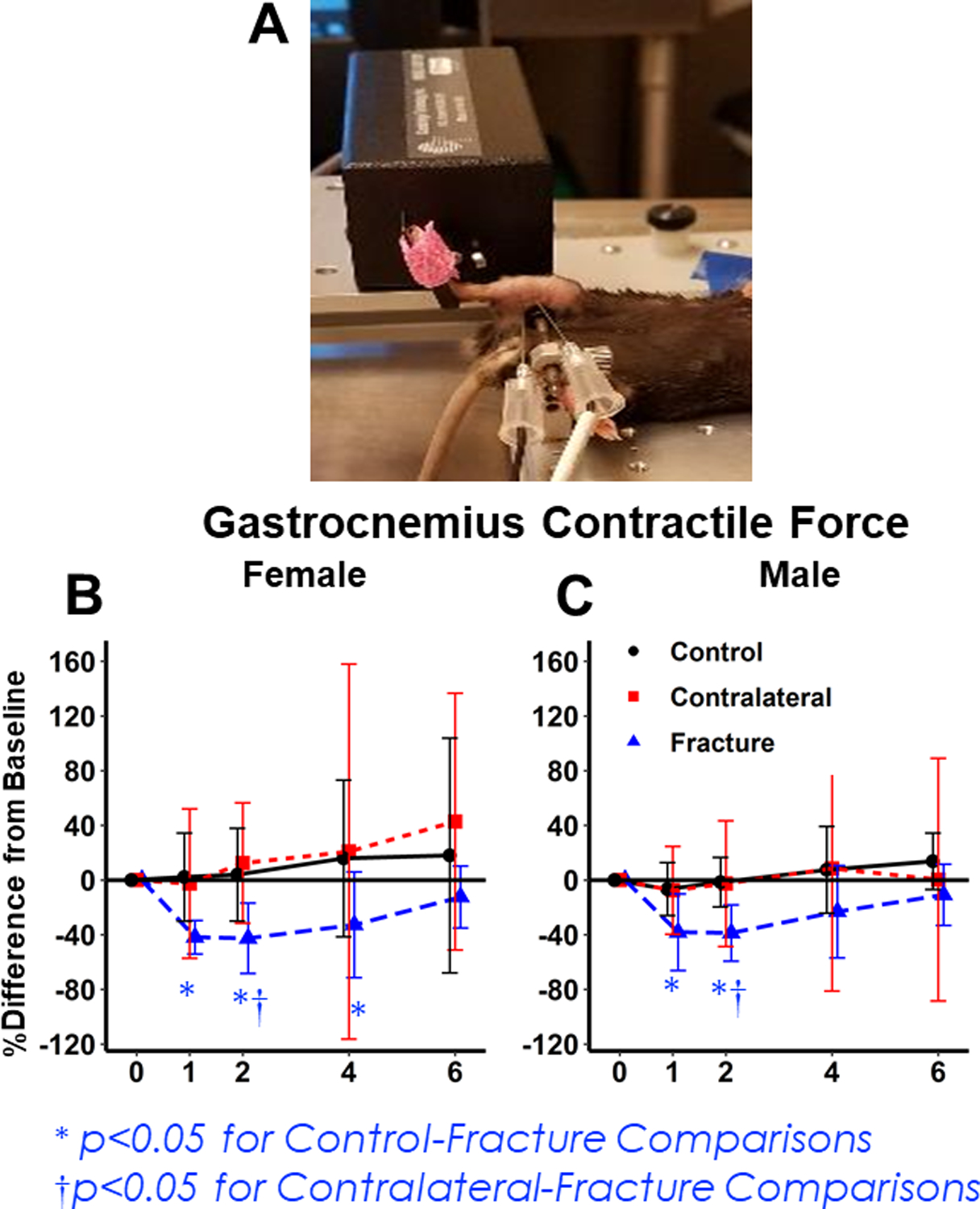
(A) Representative image of muscle analysis. (B,C) Percent change from baseline in gastrocnemius tetanic force for the Control (Uninjured), Contralateral, and Fracture Limb for Females (B) and Males (C). The injured limb shows significant decreases from baseline for 1–2 weeks post-fracture and there is no sex difference.
Muscle Mass and Histology
After euthanasia, both left and right quadriceps and the right gastrocnemius were dissected, weighed, pinned on cork at resting length, and frozen in liquid nitrogen cooled isopentane. Muscle mass was analyzed using raw values, and, to control for potential differences in muscle mass due to body size, values were also normalized to body mass. Both methods showed the same group differences, so raw values are reported. For histological analysis, 10–14 µm quadriceps cross-sections were sliced using a Leica CM 3050S cryostat and mounted on slides. Sections were washed in PBS, blocked with normal goat serum (5%), and incubated with primary laminin antibodies. After PBS wash, fluorescent secondary antibodies (Sigma) were applied for 1 h at room temperature. Digital images were taken and stitched together with a Leica DMi8 microscope and a Leica DFC9000 camera with the LAS X software. Cross-sectional fiber area (CSA) and minimum Feret diameter were measured for all fibers using the SMASH software package (Fig. 7A).31 We also calculated standard deviation for CSA and diameter for all fibers in each muscle section (hereby referred to as intra-individual variance in CSA and diameter).
Figure 7: Histological analysis of quadriceps fiber properties 2 weeks post-fracture.
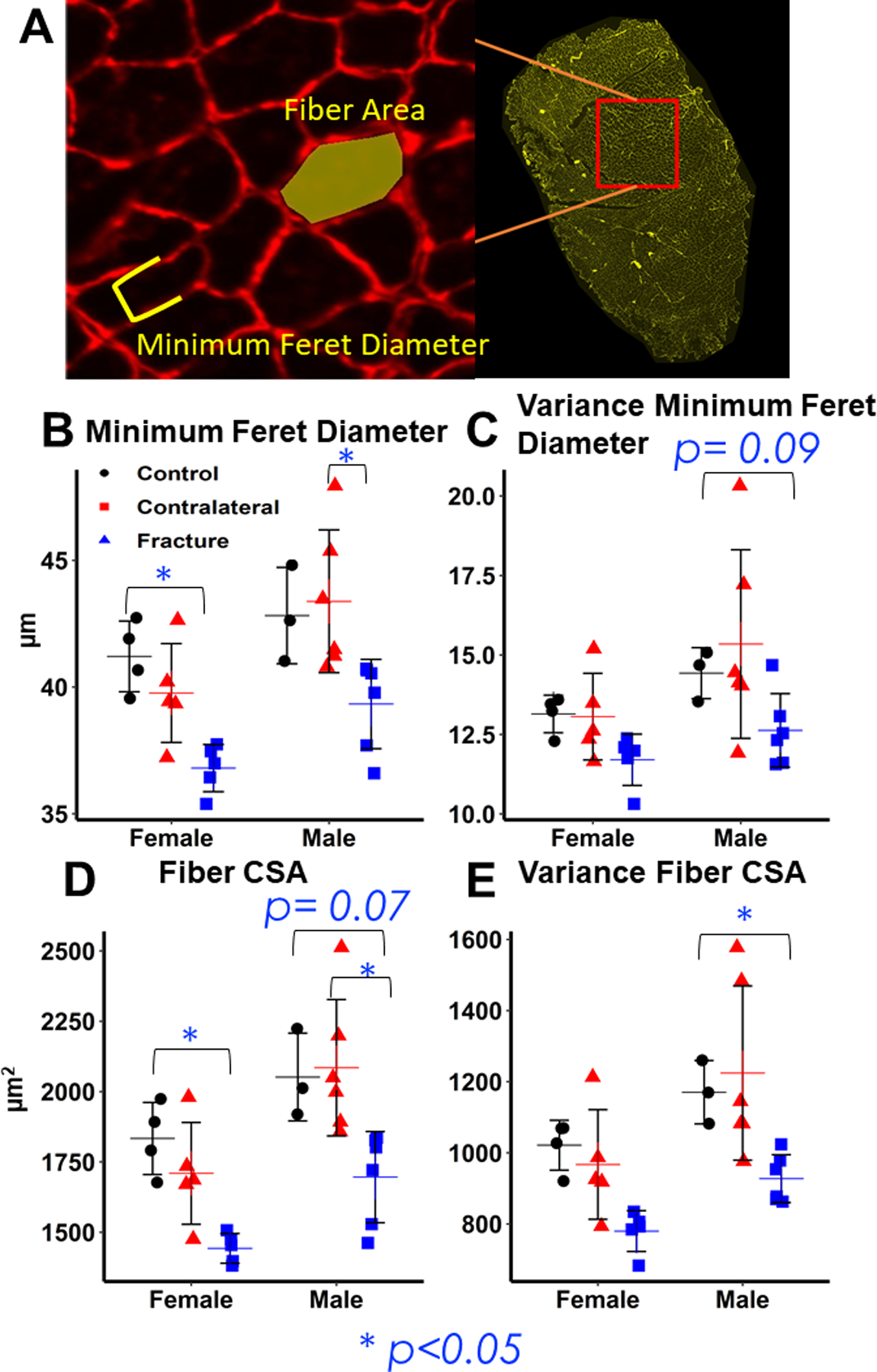
(A) Representative Image of individual muscle fiber with stain for laminin and example of minimum Feret diameter and fiber cross-sectional area measurements. (B, C) Differences in minimum Feret diameter and intra-individual variance in Feret diameter. Minimum Feret diameter and intra-individual variance is significantly lower in fracture muscle compared to control and contralateral quadriceps, but there is no difference between sexes. (D, E) Differences in fiber cross-sectional area and intra-individual variance in fiber cross-sectional area. Fiber cross-sectional area and intra-individual variance is lower in fracture muscle compared to control and contralateral quadriceps, but there is no difference between sexes.
ELISA Analysis of Inflammatory Cytokines in Serum
Twenty male and 20 female mice were euthanized 1-day post-fracture to quantify inflammatory cytokines. Immediately prior to euthanasia, mice were anesthetized via isoflurane inhalation and 0.5 to 1 ml of blood drawn via cardiac puncture. One hour after collection, blood was centrifuged at 2000 rpm at 15°C for 10 minutes. Serum was transferred to a new Eppendorf tube and stored at −80°C. Serum concentrations of IL-1β, IL-6, IL-10, and TNF-α were measured using electrochemiluminescence on a Sector Imager 2400 (Meso Scale Discovery, Rockville, MD) at the University of California Davis Mouse Biology Program.
Statistical Analysis
All results are expressed as mean ± standard deviation. Data collected at terminal end points were analyzed by two-way analysis of variance (ANOVA) stratified by sex and experimental group (fracture or control) to determine main effects and interactions. Longitudinal data (DXA and muscle force) were analyzed using repeated measures ANOVA to determine differences in the time course of outcomes. Post-hoc analyses were performed using Tukey’s Honest Significant Difference test, with p-values adjusted for multiple comparisons. Alpha-level was set at p ≤ 0.05.
Results
Dual-energy x-ray absorptiometry (DXA) analysis:
Whole-body BMC and BMD declined in both sexes two weeks after fracture. Although the two-way interaction between fracture and sex was not statistically significant, the magnitude of bone loss relative to baseline was greater in Fracture Males 2 weeks post-fracture (Fig. 1: BMD: −3.6% from baseline in Fracture Females (Fig. 1B) (p= 0.78 compared to age-matched controls);−8.1% from baseline in Fracture Males (Fig. 1C) (p= 0.02 compared to age-matched controls); BMC: −17% from baseline in Fracture Females (Fig. 1D) (p<0.001 compared to age-matched controls), −24% in Fracture Males (Fig. 1E) (p<0.001 compared to age-matched controls)). By week 4, Fracture Females were no longer significantly different from age matched controls, but Fracture Males still showed significant deficits in BMC (Fracture Females BMC: −7% difference from baseline (p= 0.195 relative to age-matched controls) (Fig. 1D); Fracture Males BMC: −9% (p= 0.02 relative to age-matched controls) (Fig. 1E)).
At the lumbar spine, BMD did not decrease in Female Fracture mice (p= 0.99) (Fig. 2B) but Male Fracture BMD decreased by 2 weeks after fracture (−8% difference from baseline (p=0.07 relative to age-matched controls (Fig. 2C). No significant differences in BMC were observed between Fracture and Control mice at the lumbar spine or femur diaphysis in either sex (Fig. 2 D,E,I,J).
Micro-computed tomography analysis of trabecular and cortical bone
Two weeks post-fracture, microstructural properties of L5 trabecular bone decreased in Fracture mice of both sexes (Fig. 3 A–D). Declines in BV/TV were similar between the sexes but Tb.Th may have declined more in males (Fracture× Sex: BV/TV: p=.291; Tb.Th: p= 0.08). Tb.Th was 13% (p<0.001) lower in Male Fractures than controls, whereas Female Fracture Tb.Th was 6% lower (p= 0.09) than controls (Fig. 3 C,D). At the contralateral distal femur, two-way interaction of Fracture and Sex was significant for Tb.Th (Fracture× Sex: BV/TV: p= 0.07; Tb.Th: p= 0.002) (Fig. 3 E–H). BV/TV was 18% lower in Fracture Males compared to Controls (p=0.038) but only 3% lower in females (p= 0.997) (Fig. 3 E,F). Similarly, Tb.Th was 11% lower in Fracture Males than Controls (p=0.008) but did not decrease in Fracture Females (Fig. 3 G,H). By 6 weeks after fracture, there were no significant differences between Fracture and Control groups of either sex at either skeletal site (Fig. 3 A–H). No significant differences were observed in trabecular separation (Tb.Sp) or trabecular number (Tb.N) between Fracture or Control mice of either sex.
Figure 3: Micro-computed tomography analysis of 5th lumbar vertebrae (L5) and distal femur trabecular bone.
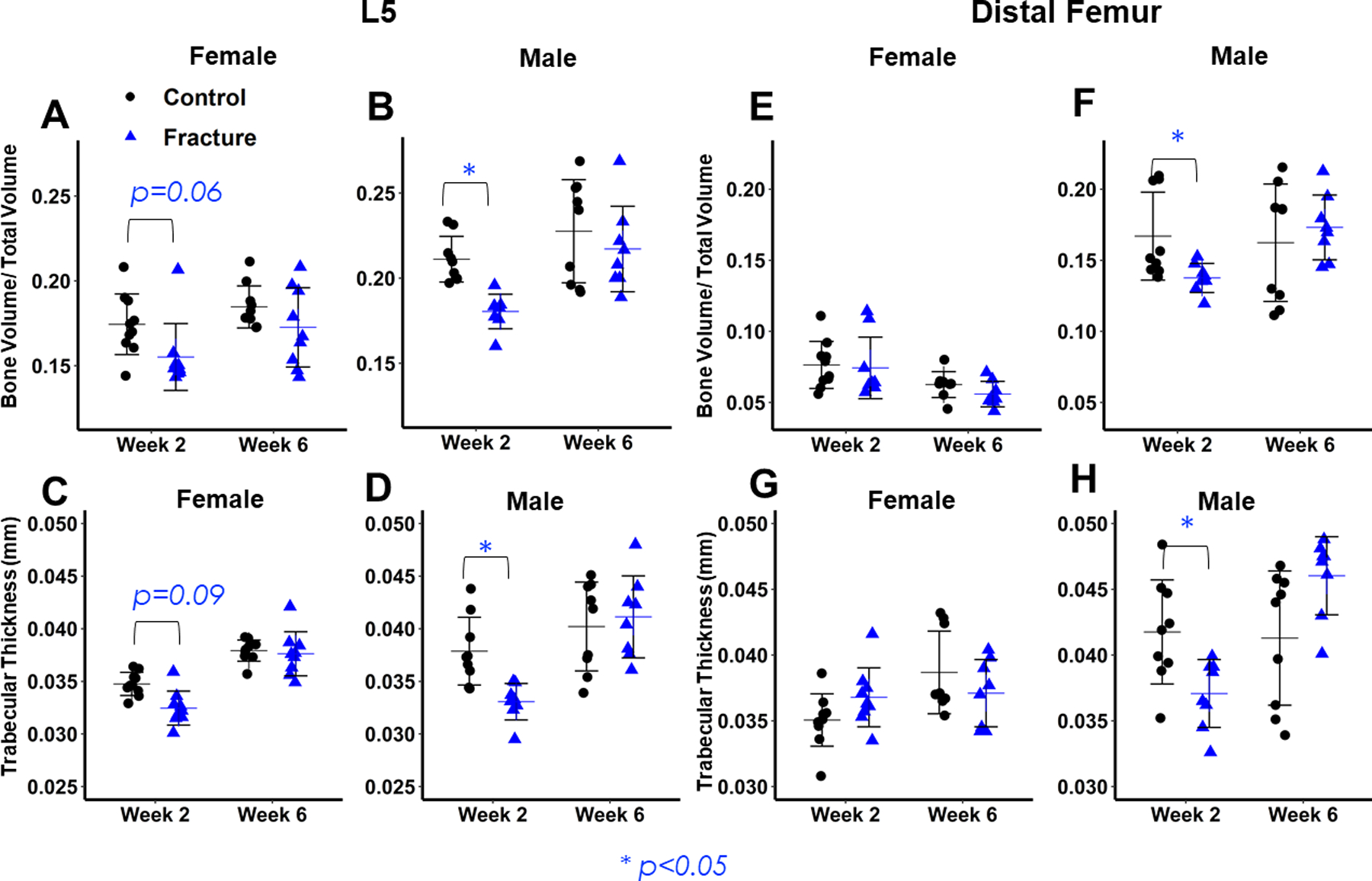
(A,B,C,D) L5 microarchitectural properties. Lower values of bone volume/ total volume (A,B) and trabecular thickness (C,D) in Fracture mice are evident in both sexes at 2 weeks post-fracture, but greater in Males. No differences between Fracture and Control mice 6 weeks post-fracture. (E,F,G,H) distal femur microarchitectural properties. Lower values of bone volume/ total volume (E,F) and trabecular thickness (G,H) in Fracture mice are evident in Males 2 weeks post-fracture, and there are no differences between Fracture and Control mice at 6 weeks post-fracture.
At the femur midshaft, cortical area exhibited a significant difference due to fracture (p= 0.035), and a nearly significant interaction between sex and fracture (p= 0.084) two-weeks post-fracture (Fig. 4 A,B). Bone area was significantly lower in Fracture Males than Controls (−8%, p=0.046) but not Females (−1%, p=0.99) (Fig. 4 A,B). Although the interaction between sex and fracture was not significant, Male Fracture mice showed greater differences compared to Controls for Polar Moment of Inertia (PMOI) and Imax (PMOI: −12%, p= 0.157; Imax: −14%, p=0.133) than Female Fracture mice ( PMOI: −4%, p=0.974; Imax: −4%, p=0.982). There were no significant differences between Fracture and Control Mice at Week 6.
Figure 4: Micro-computed tomography analysis of femur midshaft cortical bone.
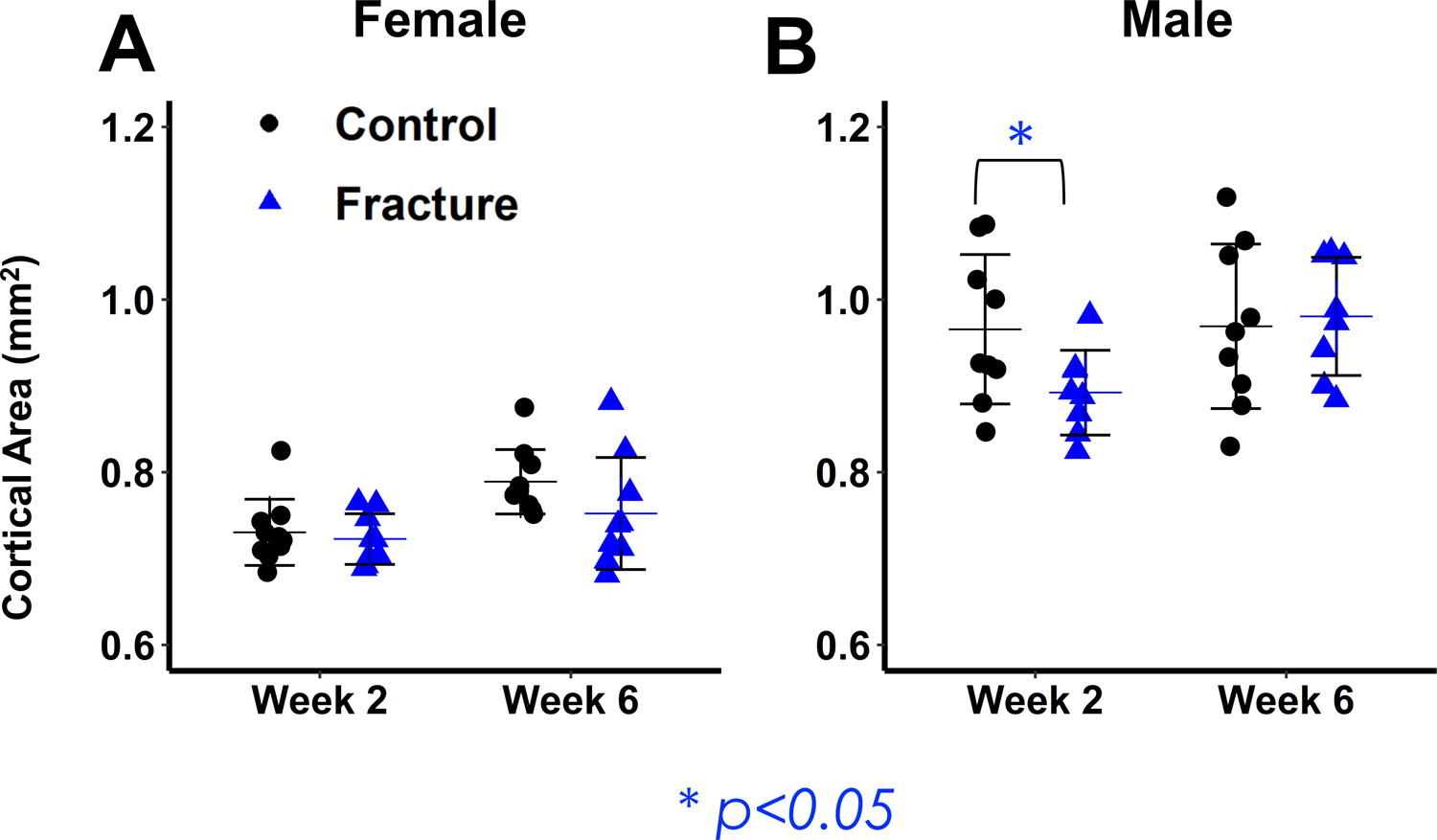
(A,B) Cortical Area. Male Fracture mice show significant decreases in cortical area at 2 weeks post-fracture and recover by 6 weeks post-fracture.
3-point bending mechanical testing
Mechanical testing of uninjured femora two weeks post-fracture detected a significant main effect of fracture in yield force (p=0.036), but the interaction between sex and fracture was not significant. At 6 weeks post-fracture, there were no significant differences in mechanical or material properties between Fracture and Control mice of either sex (Supplemental Table 1).
Muscle contractile force
In both sexes, gastrocnemius contractile force of the injured leg decreased from baseline within 1 week after fracture in the injured leg (Females: −42% decrease from baseline (p<0.001 compared to age-matched controls); Males: −38% decrease from baseline (p< 0.001 compared to age-matched Controls)), but contractile force did not decrease in Control mice or in the contralateral leg of Fracture mice (Fig. 5 B,C). The time course of contractile force changes was significantly different due to fracture but not sex. Contractile force of the Fracture Gastrocnemius was not significantly different from age-matched Controls by week 6 (Fracture Females p= 0.22; Fracture Males p= 0.20).
Muscle Mass
There was a significant decrease in quadriceps and gastrocnemius muscle mass in the fractured limb at 2 weeks post-fracture in both sexes but no significant interaction between sex and fracture. Quadriceps mass in the Facture limb was 20% lower (p=0.001) than the Control limb in female mice and 19% lower (p<0.001) in male mice; the mass of the gastrocnemius in the Fracture limb was 16% (p=0.005) lower in female mice and 14% (p=0.003) lower in male mice compared to Controls (Fig. 6 A–D). At week 6, quadriceps mass remained lower in the Fracture than the Control limb (Female: −19% (p=0.096), Male: −15% (p=0.01) (Fig. 6 A,B). However, there were no significant differences in gastrocnemius mass (Fig. 6 C,D). Contralateral muscle mass was slightly reduced relative to the Control limb at both weeks 2 and 6, though these differences were not statistically significant (Quadriceps: Wk2: Females: −5% (p=0.82), Males: −2% (p=0.405); Wk6: Females: −6% (p=0.908), Males: +1% (p= 0.976); Gastrocnemius: Wk2: Females: −5% (p=0.812); Males: −7% (p=0.275); Wk6: Females: Females: −9% (p=0.905); Males: −10% (p=0.685)) (Fig. 6 A–D).
Figure 6: Analysis of muscle mass.
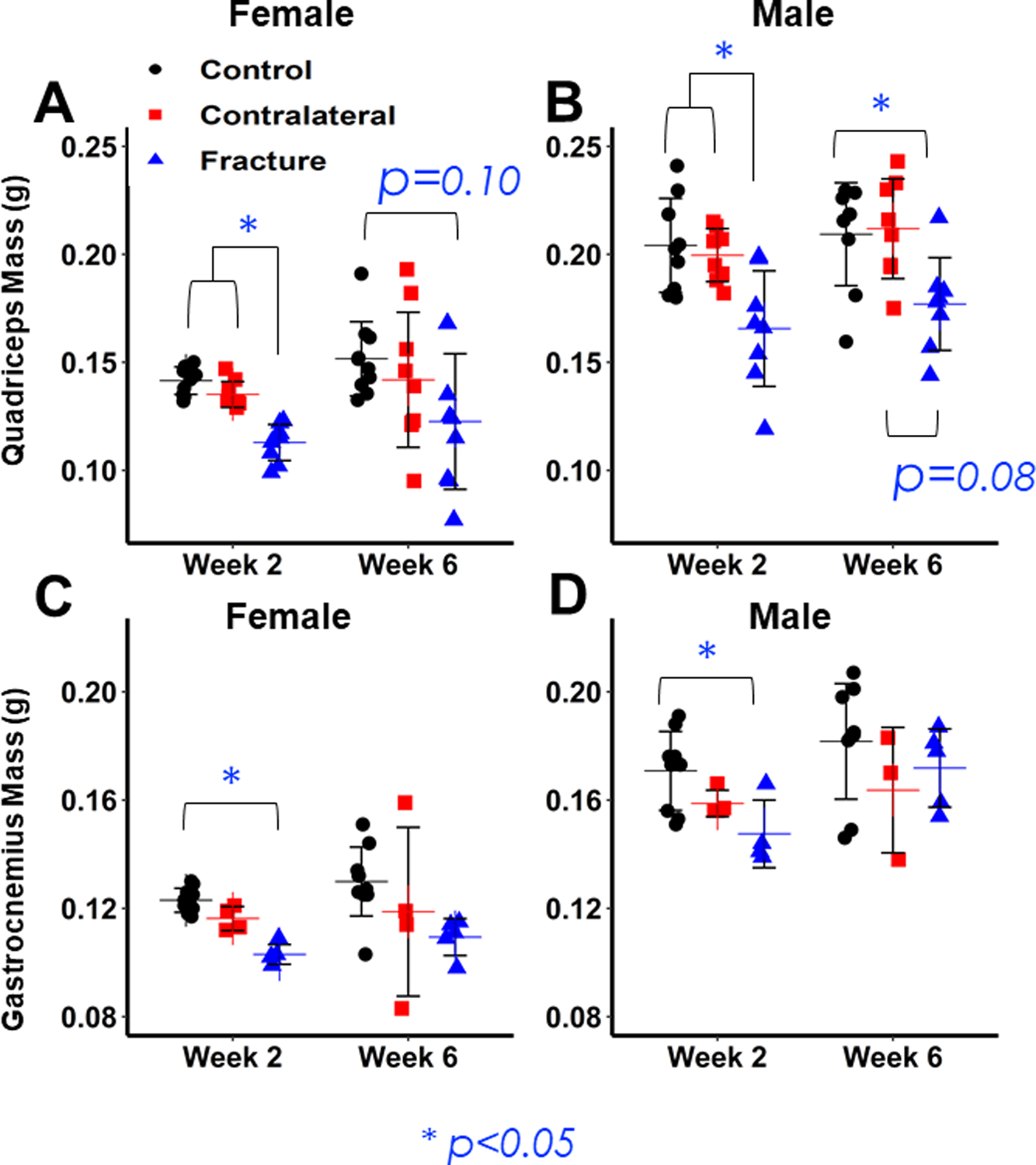
(A,B) Differences in Quadriceps mass between Control (Uninjured), Contralateral, and Fracture limbs for Females (A) and Males (B). Both sexes show significant decreases in Fracture Quadriceps mass 2 weeks post-fracture, but no difference between Control and Contralateral limbs. Fracture limb mass remains significantly lower 6 weeks post-fracture in females and there is a trend in males. (C,D) Differences in Gastrocnemius mass between Control (Uninjured), Contralateral, and Fracture limbs for Females (C) and Males (D). Both sexes show significant decreases in Fracture Gastrocnemius mass 2 weeks post-fracture, but no difference between Control and Contralateral limbs, but no differences between muscle types at Week 6.
Histological Quantification of Quadriceps Muscle Fiber Size
Two weeks post-fracture, quadriceps muscle fiber CSA and minimum Feret diameter were significantly lower in the Fracture limb compared to Controls; there was no significant interaction between sex and fracture (Fig. 7B,C). CSA was 21% lower in Fracture Females (p= 0.002 compared to Controls) and 21% lower in Fracture Males (p=0.068 compared to Controls. Minimum Feret diameter was 11% lower (p= 0.027) in the Fracture limb of female mice and 8% lower (p= 0.154) in Male mice compared to Controls. However, Contralateral fiber CSA and Feret diameter were significantly larger compared to the Fracture limb in male but not female mice (Fracture versus Contralateral CSA: Females: −16% (p=0.171), Males: −19% (p=0.016); Minimum Feret Diameter: Females: −7% (p= 0.193), Males: −9% (p= 0.016) (Fig. 7 B,C). Intra-individual variance in Minimum Feret Diameter and CSA trended lower in Fracture limbs than in Uninjured limbs, though the difference was only statistically significant in male mice (Fig. 7 D,E). Intra-individual Variance in CSA was 11% (p= 0.326) lower in Female Fracture mice compared to Controls and 18% (p= 0.016) in Male mice.
Quantification of Inflammatory Cytokines
One day after fracture, concentrations of IL-1β (p<0.001) and IL-6 (p<0.001) in serum were elevated in Fracture mice of both sexes compared to Controls (Fig. 8 A,B). The concentration of IL-1β and IL-6 was respectively 238% and 1100% higher in Fracture Females compared to Controls, and 102% and 445% higher in Fracture Males compared to Controls, with no significant interaction between fracture and sex. In contrast, TNF-α levels exhibited significant interaction between fracture and sex (p= 0.015). TNF-α was elevated by 23% in Fracture Males relative to Controls (p= 0.068), and by 21% over Fracture Females (p= 0.083), but there was no significant difference between Female Fracture and Control mice (p=0.693) (Fig. 8C). Comparison of IL-10 concentrations also detected a significant interaction between Sex and Fracture (p=0.027). While levels were highest in Fracture Males, post-hoc comparisons found no significant differences between groups (Fig. 8D).
Figure 8: Concentrations of pro-inflammatory cytokines 1-day post-fracture.
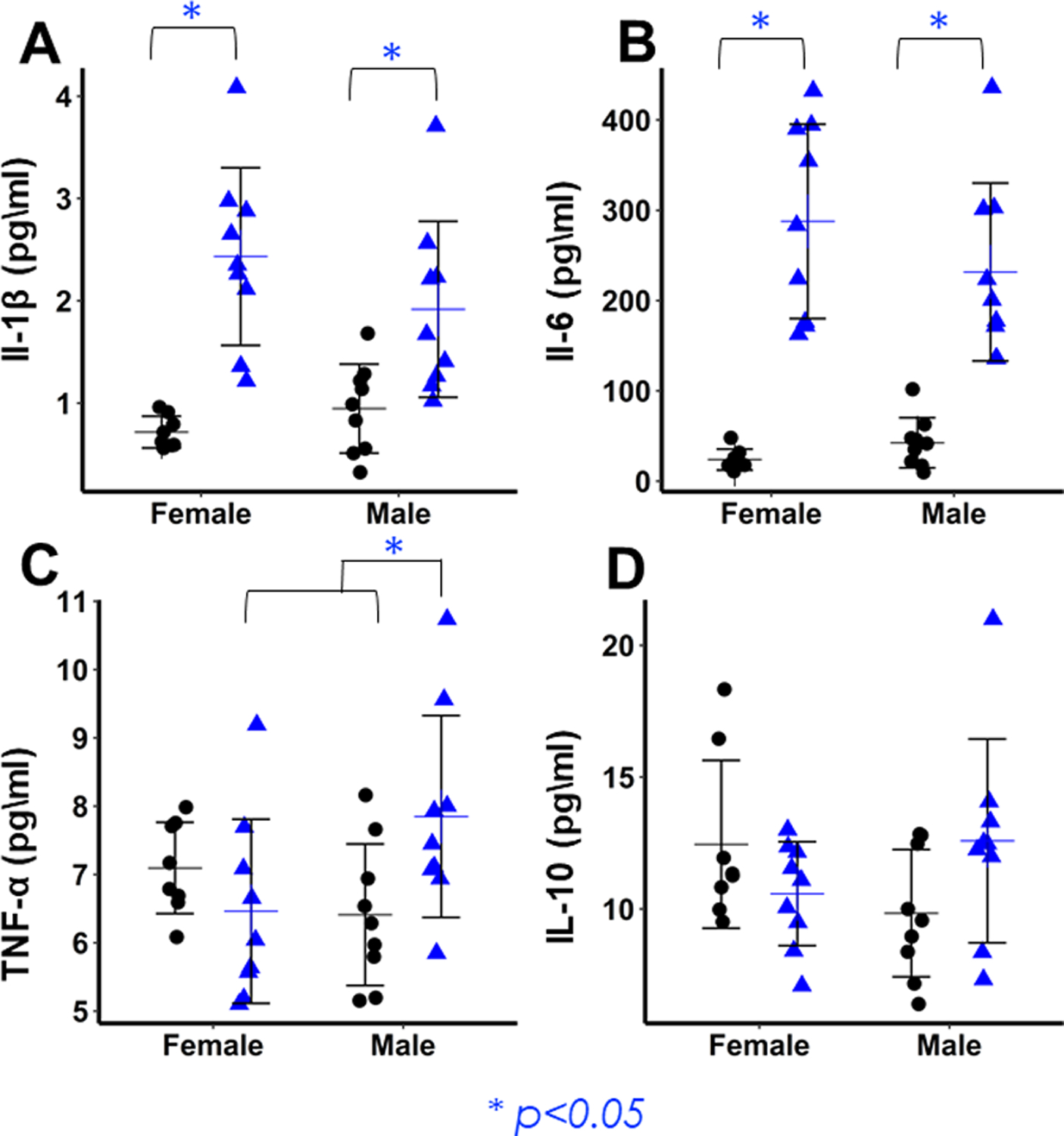
(A,B) Fracture mice of both sexes show significantly elevated levels of IL-1β (A) and IL-6 (B) relative to controls, but there are no sex differences in the magnitude of inflammation. (C) Concentrations of TNF-α are significantly elevated in Male Fracture mice relative to Male Control and Female Fracture mice. (D) IL-10 concentrations are highest in Fracture Males, but there are no significant differences between groups.
Discussion
This study investigated sex differences in systemic bone and muscle loss after femur fracture in mice. Consistent with our first hypothesis, fracture induced greater loss of bone in male mice than female mice. Whole-body BMD and BMC two weeks after fracture declined twice as much in males as females; male mice also showed greater decreases in trabecular bone volume and trabecular thickness, with a significant interaction between sex and fracture at the distal femur. Males also may have lost more cortical bone at the femur midshaft. In both sexes, recovery of bone volume was evident by 6 weeks post-fracture. Significant decreases in muscle strength, mass, and fiber properties only occurred in the fractured limb. Both sexes showed similar deficits in Fracture limb muscle fiber CSA and Feret diameter relative to uninjured limbs. Femur fracture elevated pro-inflammatory cytokine concentrations 24 hours after fracture. While IL-6 and IL-1β concentrations were elevated in Fracture mice of both sexes, and TNF-α was elevated in Male Fracture mice relative to Controls and Female Fracture mice.
This study is the first to demonstrate a sex difference in the magnitude of systemic bone loss after fracture. Males exhibited trends towards greater bone loss at both trabecular and cortical bone sites, though the interaction of fracture and sex was only statistically significant for distal femur trabecular bone properties. Even small differences in the magnitude of post-traumatic bone loss may meaningfully increase fracture risk, particularly when accounting for the entire at-risk population. Greater systemic bone loss after fracture may explain why an index fracture increases long-term risk of a subsequent fragility fracture more in males than females.8–10 Steroid hormones, especially estrogen, may drive sex differences in systemic bone loss via direct or indirect effects on bone remodeling. Estrogen inhibits bone remodeling, induces osteoclast apoptosis, and blocks osteoclast differentiation.32,33 Although osteoclast number was not evaluated in this study, it is possible that increases were greater or sustained longer in males.
Substantial recovery in BMD and bone volume in both sexes by week 6 may reflect the young age of these animals. In our prior study comparing 3-month and 12-month-old female mice, 12-month-old mice lost similar amounts of bone as young animals, but whole body BMD did not recover by 6 weeks post-fracture.11 Similarly, a fracture prior to age 20 does not correlate with future fracture risk in women, but a fracture between the ages of 20 and 50 years does, potentially due to decreased recovery from systemic bone loss with age.34 Therefore, the sex differences in systemic bone loss observed in the current study may be especially relevant in older people.
While we observed greater post-fracture systemic bone loss in males, decreases in muscle contractile force, muscle mass, and histological properties were observed only in the fractured limb. Maximal muscle loss by 1–2 weeks post-fracture and partial recovery by 6 weeks suggests a similar time-course of injury response in muscle and bone. Quadriceps muscle mass was still significantly lower at 6 weeks post-fracture, which may reflect that physical damage due to injury takes longer to heal than loss due to disuse. Studies in rats similarly found that soleus mass and fiber area decreased 2 weeks after femur fracture and began to recover by 3 weeks.35,36 Similarly, in humans, muscle force decreased by ≈60% 2-weeks post-fracture and was slow to return to post-injury values.37
Unloading likely further contributes to reductions in quadriceps and gastrocnemius mass in the fractured limb. Our prior study using female mice detected decreases in voluntary movement and activity 4 days after fracture in female mice, but not at 2, 4, and 6 weeks post-fracture.11 In the current project we observed a similar time course of bone and muscle loss, which could reflect crosstalk between these tissues or a parallel response to disuse. Bone and muscle release numerous chemical messengers known to have anabolic and catabolic effects on each other.38–40 However, further studies are needed to identify the extent of crosstalk during healing. Although we found only small non-significant decreases in Contralateral muscle mass following fracture in mice, disuse-induced systemic muscle loss is more likely to affect humans, which show systemic changes in muscle mass in both the injured limb and the whole body after fracture, potentially due to greater disuse.25,41 Low muscle mass impairs balance and increases risk of falls, potentially increasing subsequent fracture risk in both sexes.42
Interestingly, female mice exhibited possible trends toward greater decreases in muscle contractile force, mass, and fiber size in the injured limb than male mice. Although these trends were not statistically significant, these findings may indicate greater muscle loss in females following injury, either due to greater decreases in activity or a greater catabolic response in muscle. Sex differences in activity after injury have not been widely investigated, though a prior study has found that female mice experience greater declines in activity than males following restraint stress.43 Confirming these finding in our fracture model would require further investigation of sex differences in muscle loss with larger sample sizes, quantifying activity differences, and investigating other potential mechanisms.
Consistent with our hypothesis that fracture engenders systemic inflammation, serum IL-1β, IL-6, and TNF-α were elevated 1 day after fracture. Comparatively high levels of serum IL-6 and lower fold increases of IL-1β, TNF-α, and IL-10 at 1 day post-injury agree with prior studies.44–46 TNF-α and IL-1β are among the earliest pro-inflammatory molecules released after trauma, and they stimulate release of IL-6 , which remains elevated longer. 45,46
However, it remains unclear if differences in inflammation contribute to sex differences in bone loss. Higher concentrations of TNF-α seen in males may contribute to greater osteoclast activation or impairment in osteoblast function at distant skeletal sites in males.19 On the other hand, IL-6 and IL-1β concentrations did not differ between male and female fracture mice, and concentrations of IL-10, an anti-inflammatory cytokine that protects against bone resorption in chronic inflammatory conditions and inhibits osteoclast differentiation, were higher in males.47 Thus our results only partially concur with prior observations of greater inflammation in response to trauma or infection in males and estrogen deficiency. 17,18,44 However, some studies of humans and animal models found higher levels of TNF-α in male sepsis and higher IL-10 in female sepsis cases, but no sex difference in IL-6 concentrations.21,48 Overall, sex differences in the inflammatory response to trauma remain insufficiently understood. 45,46 Thus, there are some sex differences in inflammatory response at 1 day after fracture, but further studies are needed to determine the effect of acute inflammation on bone remodeling.
This study had several important limitations. First, we did not directly quantify bone remodeling or osteoclast activity using histology or serological assays. Thus, sex differences in bone remodeling post-fracture can only be inferred from chronological changes in bone structural parameters. Our prior study on age-differences in systemic bone loss after femur fracture in female mice found that fracture increased osteoclast number in the lumbar spine at 3 days post-fracture relative to control mice, but there were no differences at later time points. Therefore, it remains unclear if sex-based differences in osteoclast number and activity would have been observed at the terminal time points in this study (2 weeks and 6 weeks post-fracture). Likewise, we performed mechanical testing at only one skeletal site, and did not assess mechanical properties of skeletal sites with high trabecular bone content. Also, we only examined 3-month-old mice, which recovered from systemic bone loss within 6 weeks, whereas older animals may show a reduced ability to recover from bone loss.6 Our previous study showed that whole-body BMD did not recover to baseline values in 1 year old female mice (roughly equivalent to middle-aged women) by 6 weeks post-fracture. Therefore, it is possible that sex differences are of a greater magnitude or longer lasting in older animals. Despite these limitations, investigating systemic bone loss and recovery in young mice was a logical first step to investigate whether sex-dependent differences in bone loss were detectable and whether male mice were able to recover as efficiently as female mice. Future studies can be specifically designed to identify specific mechanisms responsible for sex differences. To minimize the number of animals used, we only evaluated inflammation at a single time point, and a 1 day time point was a reasonable choice based on prior work.11,44 However, cytokine expression varies in complex ways during fracture healing and sex differences may manifest at other time points.49 Despite these limitations, our study has several important strengths. We collected longitudinal data for both bone and muscle, and we detected sex differences in bone loss after fracture using both DXA and μCT analysis of bone microstructure. Analysis of muscle also found clear evidence that muscle atrophy is most pronounced in muscles directly surrounding the fractured bone, and the magnitude of muscle loss does not vary by sex to the same degree as bone loss.
Conclusion
In this study, we showed that young male mice experienced greater systemic bone loss than females after fracture. Conversely, no clear sex differences in muscle loss after fracture were observed, and muscle changes were predominantly confined to the injured limb. Inflammation may vary due to sex, but the results do not concretely support a role for inflammation in post-fracture systemic bone loss. Overall, greater post-fracture bone loss in males may be at least partly responsible for an increased risk of subsequent fracture following an initial fracture in men. Determining the etiology of sex differences in bone loss after fracture, potentially focusing on the role of sex hormones, could help identify specific populations at risk of systemic bone loss following fracture, and could inform treatments aimed at preserving skeletal health.
Supplementary Material
Acknowledgements:
We thank James Graham for performing Enzyme Linked Immunosorbent Assays at the University of California Davis Mouse Biology Program MMPC (NIH grant DK092993). We also thank Dr. Lucas Smith at the University of California Davis for his assistance with histological imaging and access to microscopy equipment and software.
Research reported in this publication was supported by National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health (NIH) under award number R01AR071459. The project described was also supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant number UL1 TR001860 and linked award TL1 TR001861. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Laurent M, Gielen E, Claessens F, et al. 2013. Osteoporosis in older men: Recent advances in pathophysiology and treatment. Best Pract. Res. Clin. Endocrinol. Metab 27(4):527–539. [DOI] [PubMed] [Google Scholar]
- 2.Frost M, Wraae K, Abrahamsen B, et al. 2012. Osteoporosis and vertebral fractures in men aged 60–74 years. Age Ageing 41(2):171–177. [DOI] [PubMed] [Google Scholar]
- 3.Adler RA. 2018. Update on osteoporosis in men. Best Pract. Res. Clin. Endocrinol. Metab 32(5):759–772. [DOI] [PubMed] [Google Scholar]
- 4.Burge R, Dawson-Hughes B, Solomon DH, et al. 2007. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J. Bone Miner. Res 22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 5.Johnell O, Kanis JA, Odén A, et al. 2004. Fracture risk following an osteoporotic fracture. Osteoporos. Int 15(3):175–179. [DOI] [PubMed] [Google Scholar]
- 6.Osipov B, Emami AJ, Christiansen BA. 2018. Systemic bone loss after fracture. Clin. Rev. Bone Miner. Metab 16:116–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Christiansen BA, Harrison SL, Fink HA, et al. 2018. Incident fracture is associated with a period of accelerated loss of hip BMD: the study of osteoporotic fractures. Osteoporos. Int 29:2201–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haentjens P, Autier P, Collins J, et al. 2003. Colles fracture, spine fracture, and subsequent risk of hip fracture in men and women: a meta-analysis. J. Bone Jt. Surg.-Am Vol. 85(10):1936–1943. [DOI] [PubMed] [Google Scholar]
- 9.Amin S, Melton LJ, Achenbach SJ, et al. 2013. A distal forearm fracture in childhood is associated with an increased risk for future fragility fractures in adult men, but not women. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res 28(8):1751–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson CM, Royds M, Abraham A, et al. 2002. Refractures in patients at least forty-five years old: a prospective analysis of twenty-two thousand and sixty patients. J. Bone Jt. Surg.-Am Vol. 84(9):1528–1533. [DOI] [PubMed] [Google Scholar]
- 11.Emami AJ, Toupadakis CA, Telek SM, et al. 2019. Age Dependence of Systemic Bone Loss and Recovery Following Femur Fracture in Mice. J. Bone Miner. Res 34(1):157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Redlich K, Smolen JS. 2012. Inflammatory bone loss: pathogenesis and therapeutic intervention. Nat. Rev. Drug Discov 11(3):234–250. [DOI] [PubMed] [Google Scholar]
- 13.Loi F, Córdova LA, Pajarinen J, et al. 2016. Inflammation, fracture and bone repair. Bone 86:119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weckbach S, Perl M, Heiland T, et al. 2012. A new experimental polytrauma model in rats: molecular characterization of the early inflammatory response. Mediators Inflamm 2012:890816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choy E 2008. Inhibiting interleukin-6 in rheumatoid arthritis. Curr. Rheumatol. Rep 10(5):413–417. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt EM, Davies M, Mistry P, et al. 2013. Selective blockade of tumor necrosis factor receptor I inhibits proinflammatory cytokine and chemokine production in human rheumatoid arthritis synovial membrane cell cultures. Arthritis Rheum 65(9):2262–2273. [DOI] [PubMed] [Google Scholar]
- 17.Scotland RS, Stables MJ, Madalli S, et al. 2011. Sex differences in resident immune cell phenotype underlie more efficient acute inflammatory responses in female mice. Blood 118(22):5918–5927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bullard MK, Bir N, Kwan R, et al. 2010. Women rule. Surgery 147(1):134–137. [DOI] [PubMed] [Google Scholar]
- 19.Pacifici R 1996. Estrogen, cytokines, and pathogenesis of postmenopausal osteoporosis. J. Bone Miner. Res 11(8):1043–1051. [DOI] [PubMed] [Google Scholar]
- 20.Diodato MD, Knöferl MW, Schwacha MG, et al. 2001. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine 14(3):162–169. [DOI] [PubMed] [Google Scholar]
- 21.Schroder J, Kahlke V, Staubach K-H, Zabel P. 1998. Gender differences in human sepsis. Arch. Surg 133:1200–1205. [DOI] [PubMed] [Google Scholar]
- 22.Kovacs EJ, Plackett TP, Witte PL. 2004. Estrogen replacement, aging, and cell-mediated immunity after injury. J. Leukoc. Biol 76(1):36–41. [DOI] [PubMed] [Google Scholar]
- 23.Ham DJ, Kennedy TL, Caldow MK, et al. 2015. Citrulline does not prevent skeletal muscle wasting or weakness in limb-casted mice. J. Nutr 145(5):900–906. [DOI] [PubMed] [Google Scholar]
- 24.Lynch GS, Schertzer JD, Ryall JG. 2007. Therapeutic approaches for muscle wasting disorders. Pharmacol. Ther 113(3):461–487. [DOI] [PubMed] [Google Scholar]
- 25.Wall BT, Dirks ML, Snijders T, et al. 2014. Substantial skeletal muscle loss occurs during only 5 days of disuse. Acta Physiol. Oxf. Engl 210(3):600–611. [DOI] [PubMed] [Google Scholar]
- 26.Rosa-Caldwell ME, Greene NP. 2019. Muscle metabolism and atrophy: let’s talk about sex. Biol. Sex Differ 10(1):43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toupadakis CA, Wong A, Genetos DC, et al. 2012. Long-term administration of AMD3100, an antagonist of SDF-1/CXCR4 signaling, alters fracture repair. J. Orthop. Res 30(11):1853–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonnarens F, Einhorn TA. 1984. Production of a standard closed fracture in laboratory animal bone. J. Orthop. Res 2(1):97–101. [DOI] [PubMed] [Google Scholar]
- 29.Bouxsein ML, Boyd SK, Christiansen BA, et al. 2010. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. J. Bone Miner. Res 25(7):1468–1486. [DOI] [PubMed] [Google Scholar]
- 30.Jepsen KJ, Silva MJ, Vashishth D, et al. 2015. Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J. Bone Miner. Res 30(6):951–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith LR, Barton ER. 2014. SMASH – semi-automatic muscle analysis using segmentation of histology: a MATLAB application. Skelet. Muscle 4(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khosla S, Oursler MJ, Monroe DG. 2012. Estrogen and the skeleton. Trends Endocrinol. Metab. TEM 23(11):576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khosla S 2013. Pathogenesis of age-related bone loss in humans. J. Gerontol. A. Biol. Sci. Med. Sci 68(10):1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu F, Mason B, Horne A, et al. 2002. Fractures between the ages of 20 and 50 years increase women’s risk of subsequent fractures. Arch. Intern. Med 162(1):33. [DOI] [PubMed] [Google Scholar]
- 35.Inoue M, Sakai Y, Oe K, et al. 2020. Transcutaneous carbon dioxide application inhibits muscle atrophy after fracture in rats. J. Orthop. Sci 25(2):338–343. [DOI] [PubMed] [Google Scholar]
- 36.Hofman M, Kolejewska A, Greven J, et al. 2020. Gait analysis and muscle weight analysis after lower extremity fractures in a small animal model. Gait Posture 77:207–213. [DOI] [PubMed] [Google Scholar]
- 37.Gaston P, Will E, McQueen MM, et al. 2000. Analysis of muscle function in the lower limb after fracture of the diaphysis of the tibia in adults. J. Bone Joint Surg. Br 82(3):326–331. [DOI] [PubMed] [Google Scholar]
- 38.Maurel D, Jähn K, Lara-Castillo N. 2017. Muscle–bone crosstalk: emerging opportunities for novel therapeutic approaches to treat musculoskeletal pathologies. Biomedicines 5(4):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han W, He W, Yang W, et al. 2016. The osteogenic potential of human bone callus. Sci. Rep 6:36330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Regan JN, Trivedi T, Guise TA, Waning DL. 2017. The role of TGFβ in bone-muscle crosstalk. Curr. Osteoporos. Rep 15(1):18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fox KM, Magaziner J, Hawkes WG, et al. 2000. Loss of bone density and lean body mass after hip fracture. Osteoporos. Int 11(1):31–35. [DOI] [PubMed] [Google Scholar]
- 42.Wong RMY, Wong H, Zhang N, et al. 2019. The relationship between sarcopenia and fragility fracture—a systematic review. Osteoporos. Int 30(3):541–553. [DOI] [PubMed] [Google Scholar]
- 43.Yamaura K, Bi Y, Ishiwatari M, et al. 2013. Sex differences in stress reactivity of hippocampal BDNF in mice are associated with the female preponderance of decreased locomotor activity in response to restraint stress. Zoolog. Sci 30(12):1019–1024. [DOI] [PubMed] [Google Scholar]
- 44.Haffner-Luntzer M, Fischer V, Prystaz K, et al. 2017. The inflammatory phase of fracture healing is influenced by oestrogen status in mice. Eur. J. Med. Res 22:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kobbe P, Vodovotz Y, Kaczorowski DJ, et al. 2008. Patterns of cytokine release and evolution of remote organ dysfunction after bilateral femur fracture. Shock 30(1):43–47. [DOI] [PubMed] [Google Scholar]
- 46.Chow CC, Clermont G, Kumar R, et al. 2005. The acute inflammatory response in diverse shock states. Shock 24(1):74–84. [DOI] [PubMed] [Google Scholar]
- 47.Lampiasi N, Russo R, Zito F. 2016. The alternative faces of macrophage generate osteoclasts. BioMed Res. Int 2016:9089610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Drechsler S, Weixelbaumer K, Raeven P, et al. 2012. Relationship between age/gender-induced survival changes and the magnitude of inflammatory activation and organ dysfunction in post-traumatic sepsis. PloS One 7(12):e51457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baht GS, Vi L, Alman BA. 2018. The role of the immune cells in fracture healing. Curr. Osteoporos. Rep 16(2):138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


