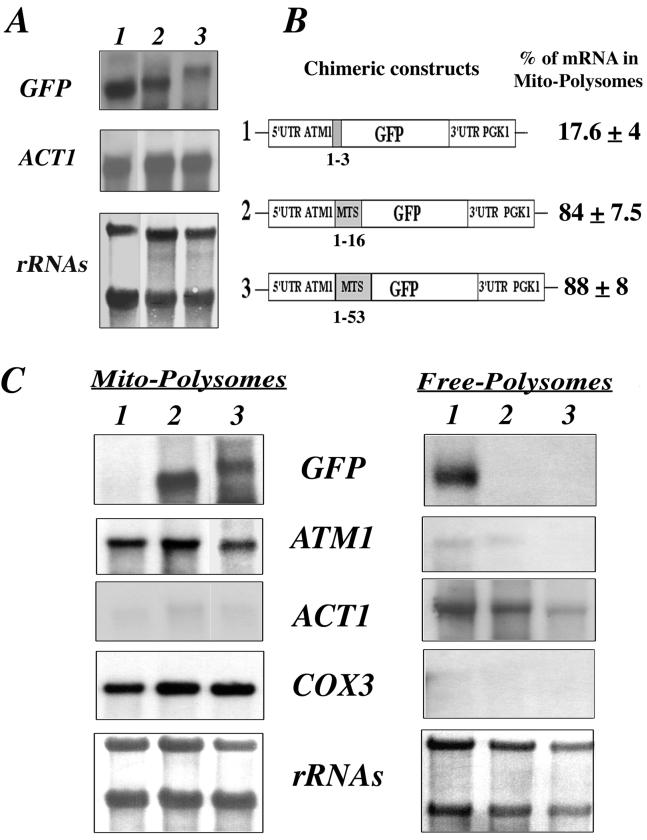
FIG. 5.
Minimal region within the mts of Atm1p required for the correct localization of GFP hybrid mRNAs. (A) Three plasmids in which the GFP was fused in frame either with the complete mts of Atm1p or with shortened mts versions were obtained. RNA extracted from whole cells was checked by Northern blot analysis. The steady-state levels of each hybrid mRNA were very similar, and the sizes measured were as expected. The approximate size for each individual mRNA was as follows: plasmid 1, 0.9 kb; plasmid 2, 1 kb; and plasmid 3, 1.1 kb. (B) Chimeric constructs examined are represented. In plasmid 1 the GFP was fused in frame to the first three amino acids of Atm1p, in plasmid 2 the first 16 amino acids of Atm1p were fused to the GFP, and in plasmid 3 the complete mts was present. In all of the plasmids, the stop codon was associated with the 3′UTR of the PGK1 gene. Calculations of the relative abundance of each mRNA in mitochondrion-bound polysomes, for six independent experiments, were obtained after the normalization of each signal with the internal markers COX3 or ACT1. (C) Northern blots performed with RNAs purified from mitochondrion-bound polysomes (Mito-Polysomes) and free cytoplasmic polysomes (Free-Polysomes) are shown. Methylene blue staining of the filters is shown at the bottom. The autoradiograms represent exposures times of between 2 and 4 h at −80°C, with Amersham intensifying screens, for all of the probes except ATM1, which required an exposure time of approximately 16 h.