Abstract
Contamination of agricultural soil by chromium (Cr) is a serious menace to environmental safety and global food security. Although potential of salicylic acid (SA) in mitigating heavy metal (HM) toxicity in plants is well recognized, detailed physiological mechanisms behind such beneficial effects under Cr-stress in tomato (Solanum lycopersicum L.) plant are far from being completely unravelled. The present study evaluated the efficacy of exogenously applied SA, in alleviating Cr-mediated alterations on photosynthesis and antioxidant defense in tomato exposed to three different concentrations of Cr(VI) [0, 50, and 100 mg Cr(VI) kg−1 soil]. Exposure of tomato plants to Cr resulted in increased Cr-accumulation and oxidative damage, as signposted by high Cr concentration in root as well as shoot, augmented malondialdehyde (MDA) and superoxides levels, and inhibition in enzymes of ascorbate–glutathione (AsA-GSH) cycle. Furthermore, a significant (P ≤ 0.05) reduction in photosynthetic pigments and gas exchange parameters was also evident in Cr-stressed tomato plants. Findings of the present study showed that exogenous application of 0.5 mM SA not only promoted plant growth subjected to Cr, but also restored Cr-mediated disturbances in plant physiology. A significant (P ≤ 0.05) decrease in Cr acquisition and translocation as evidenced by improved growth and photosynthesis in SA-treated plants was observed. Additionally, exogenous SA application by virtue of its positive effect on efficient antioxidant system ameliorated the Cr-mediated oxidative stress in tomato plants as signposted by lower MDA and superoxide levels and improved AsA-GSH cycle. Overall, current study advocates the potential of exogenous SA application in amelioration of Cr-mediated physiological disturbances in tomato plant.
Keywords: Ascorbate–glutathione cycle, Chromium (VI), Oxidative stress, Photosynthesis, Salicylic acid, Tomato
Introduction
Sustainable agriculture and providing safe, sufficient, and nutritious agricultural products is the need of the hour. However, growth, development, and productivity of crops are negatively affected by abiotic stresses such as heavy metal (HM) pollution. Elements with atomic number more than 20 and mass of about 5 g cm−3 or more are generally considered as HMs (Alloway 2012). Some HMs are essential at very low levels like copper (Cu), iron (Fe), chromium (Cr), zinc (Zn), and selenium (Se), whereas others do not have any biological role in plants like arsenic (As), lead (Pb), mercury (Hg). Rapid industrialization and intensification of anthropogenic activities has amplified the rate and amount of HM pollution in the environment (Sarath and Puthur 2020; Shackira et al. 2021). In the past 5 decades, tons of HMs have been released into the environment globally, most of which have accumulated in soil, and thus caused serious HM pollution (Chen et al. 2016). Unprecedented bio-magnification and bio-accumulation of HMs in the environment have become a dilemma to living organisms including plants. Contamination of agricultural soil and ground water by toxic HMs such as arsenic (As), cadmium (Cd), mercury (Hg), chromium (Cr), and lead (Pb) could negatively affect plants growth and productivity, which ultimately affect human health through food chain (Nguyen et al. 2021; Sameena and Puthur 2021). Exposure of plants to HMs results in global crop yield reduction that is worsening the dwindling equilibrium between crop production and exponential population growth (Singh et al. 2016a, b).
Chromium (Cr) has wide usage applications in steel, leather, textile, painting, electroplating, and other industries leading to contamination of soil and ground water (Ali et al. 2015). China, European countries, and India are major Cr contaminated countries in the world (Gao and Xia 2011; Kazakis et al. 2017; Kanagaraj and Elango 2019; Tomoloet et al. 2020). Chromium concentrations as high as 350 mg kg−1 has been reported in contaminated agricultural soils, which is much higher than the maximum permissible limit of 200 mg kg−1 of Cr in soil (Adagunodo et al. 2018; Srivastava et al. 2021). Contamination of agricultural soil with Cr is a prime source of Cr entering our food chain (Rebhi et al. 2019). Moreover, irrigation of crops with raw tannery effluents which contain HMs like Cr further aggravates Cr accumulation in crops that ultimately causes serious health hazard (Sharma et al. 2020). Beside entering in food chain and affecting human health, presence of Cr in plants adversely affect their physiology and metabolism (Ahmad et al. 2019; Singh et al. 2020).
Chromium exists predominantly as Cr(III) and Cr(VI) in the environment (Srivastava et al. 2021). Among the two forms, Cr(VI) is more mobile and is severely phytotoxic as compared to Cr(III) (Wakeel et al. 2020). In plants, Cr(VI) cause toxicities at morphological, physiological, metabolic, and molecular levels (Gomes et al. 2017) and adverse effects of Cr(VI) on plant root and shoot has been well reported (Shahid et al. 2017; Srivastava et al. 2021). Presence of Cr(VI) in plant cell has been reported to compromise leaf gas attributes and photosynthetic pigments in plants (Hossain et al. 2018). Additionally, Cr-mediated ultrastructural alterations in plant parts and oxidative stress through over-production of reactive oxygen species (ROS) is another effect of Cr(VI), that compromise plant performance under stress (Eleftheriou et al. 2015; Hossain et al. 2018; Singh et al. 2020). These excessive ROS, if not, efficiently scavenged by the plants defense system, initiate lipid peroxidation and cause damage to DNA, proteins, cellular membranes and organelles (García-Caparróset al. 2020).
Plant cells are equipped with efficient defense mechanisms to scavenge excessive ROS generated due to Cr(VI) toxicity. Ascorbate–glutathione (AsA-GSH) cycle is one such efficient antioxidant system for removing excess ROS generated under stress conditions in plants (Shen et al. 2018). The AsA-GSH cycle involves superoxide dismutase (SOD) which dismutates the superoxide radical (O2.−) to either molecular oxygen or to H2O2, whereas ascorbate peroxidase (APX) catalyzes the reduction of H2O2 to produce monodehydroascorbate (MDHA), using ascorbate (AsA) as a donor of electron. This MDHA reduced back to AsA by monodehydroascorbate reductase (MDHAR). Dehydroascorbatereductase (DHAR) utilizes glutathione (GSH) to reduce dehydroascorbate (DHA), previously metabolized from MDHA. Concurrently, GSH gets oxidized to glutathione disulfide (GSSG) by DHAR and the GSSG, finally reduced back to GSH by glutathione reductase (GR) using NADPH (Foyer and Noctor 2011).
Tomato (Solanum lycopersicum L.) is the second most important crop next to potato, and it is widely cultivated throughout the world (Celma et al. 2009; Global Tomato Industry Report, 2020). Tomato plays an important role in daily diet possessing high content of dietary fibers, vitamins, and antioxidants (Sumalan et al. 2020; Tan et al. 2021). Considering the importance of tomato as an important agricultural crop, it is imperative to understand and device a mechanism(s) to foil the negative consequences on its productivity, if grown under Cr contaminated soil. Salicylic acid (SA), a potent plant hormone, plays a significant role in augmenting a/biotic stress tolerance in plants (El-Dakak and Hassan 2020; Zewail et al. 2020). It significantly promotes plant growth by regulating a myriad of physiological processes involving synthesis of osmolytes, enhancing antioxidants, and balance of mineral status in plants (Mohamedet al. 2020). It has also been reported to modulate activities of anti-stress enzymes such as catalase (CAT), peroxidase, and SOD and alleviates Cr-mediated cell membrane damage (Islam et al. 2016). It is clear that Cr(VI) severely affects the tomato productivity and it could be managed by exogenous SA supplementation. Therefore, the present study tries to unravel some offsetting effects of exogenous SA in tomato plant grown under Cr(VI) stress by: (i) quantifying Cr(VI) accumulation in root and shoot; (ii) assessing the damage caused by Cr(VI) on leaf epidermis and guard cells; (iii) analyzing the leaf gas exchange parameters; (iv) quantifying the degree of oxidative damage; and (v) evaluating the efficiency of AsA-GSH cycle.
Material and methods
Experimental layout
Tomato variety Pusa Ruby was used in the study. It is an early growing cultivar with an average yield of 32.5 t ha−1. Seeds were procured from Indian Agricultural Research Institute (IARI), New Delhi, India. The study consisted of two phases: (i) dose selection of Cr(VI) and SA; and (ii) determination of exogenous SA-mediated ameliorative effects on tomato grown under Cr(VI) stress. For the selection of Cr(VI) doses, seed germination test was performed with different Cr(VI) concentrations (0, 50, 100, 200, and 250 mg L−1) in Petri dishes. The result revealed that at doses more than 100 mg L−1of Cr(VI), tomato seeds failed to germinate. Therefore, further study continued with three concentrations of Cr(VI) viz. control (0 mg Cr kg−1 soil), moderate (50 mg Cr kg−1 soil), and high (100 mg Cr kg−1 soil). These Cr(VI) doses are in conformity with maximum permissible limit of Cr in soil as prescribed by Adagunodo et al. (2018) and are also in accordance with previous report of Zewail et al. (2020). For SA dose selection, seeds were germinated in Petri dishes having moist germination sheets. Different concentrations of SA viz. 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mM were screened for dose selection. Among different concentrations of SA, 0.5 mM SA dose showed the highest seed germination percentage (93.33%), so this concentration was selected as the optimum SA concentration to carry out the experiments further.
Chromium (VI) and SA exposure to plants
Soil (0–30 cm) collected from the Botanical garden, University of Delhi was mixed with manure in 3:1 ratio, air-dried and used as soil material for growing plants. Before applying Cr treatment, the soil was analyzed for Cr concentration and contained 2.73 ± 0.46 mg Cr(VI) kg−1 soil. The ECe and pH of the soil used in the study was 2.5 dS m−1 and 6.8, respectively. Soil material was then manually contaminated with Cr(VI) in plastic trays by adding two different doses of Cr(VI) [50 and 100 mg Cr(VI) kg−1 soil−] using K2Cr2O7 and soil material without Cr(VI) served as control. Thoroughly mixed soil then kept for four weeks under natural conditions to allow proper equilibration of Cr(VI) in soil. Ten surface sterilized seeds were evenly sown in plastic pots (10 × 10 × 8 cm, each pot having 250 g soil, Fig. 1) and each treatment consisted of three replicates. Plants were grown for 30 days in a plant growth chamber (Daihan Labtech Co. Ltd.) under controlled temperature (22 ± 2 ºC) and illuminated (14 h day) with light intensity of 200 μE m−2 s−1. After 30 days, SA solution (prepared in 0.05% tween-20) was sprayed twice after one-week interval, so that each leaf get completely drenched with SA. Afterwards, fully developed young leaves on upper nodes of the plant were harvested (45 days of growth) during sunny day and immediately placed in liquid nitrogen and stored at −80 ºC for analysis of biochemical parameters. However, for the analysis of AsA and GSH content, fully developed mature leaves (just below the fully developed young leaves) of the plant were taken and assay was carried out immediately as these metabolites change rapidly in the plants.
Fig. 1.
Image showing the tomato plants in plastic pots grown under various Cr(VI) concentrations with and without SA (0.5 mM)
Chromium (VI) quantification
Plants were carefully uprooted, washed, and separated into roots and shoots followed by oven-drying at 60 ºC. Dried plant sample (1 g) was powdered, sieved (0.5-mm sieve) and digested with nitric acid and hydrogen peroxide (8:10; v/v), in Kjeldahl tubes. After digestion, the solutions were cooled, filtered, and diluted to 50 mL using DDW (APHA 2005). Atomic absorption spectrophotometer (AAS) (Perkin-Elmer A analyst 600) was used for quantification of Cr in the digested solutions. Similarly, soil samples were digested in a HNO3:HClO4 solution (3:1; v/v), filtered through Whatman filter paper number 1, and used for Cr(VI) determination using AAS. The plant uptake factor was calculated as-
Leaf epidermis analysis by scanning electron microscope
Scanning electron microscopy was done to analyze the leaf epidermis and stomatal morphology. Leaf sections (1 mm2) was fixed in 25% glutaraldehyde and 1% p-formaldehyde fixative and passed through ethanol gradient series of 25%, 50%, 75%, and 100% (30 min in each) for critical point drying (Yuan et al. 2012). Samples were then mounted on carbon stubs followed by gold-palladium coating, and finally observed for any anomalies in the epidermal cells as well as guard cells under a scanning electron microscope (JEOL-JSM-6610LV).
Leaf gas exchange parameters analysis
The leaf gas exchange parameters such as net photosynthetic rate (A), transpiration rate (E), and stomatal conductance (GH2O) were done on fully expanded young leaves between 9 and 12 am on a clear sunny day using an infrared gas analyzer (IRGA, GFS-3000 Portable Photosynthesis System; Walz). Before taking observations, the instrument was calibrated for leaf surface area (3 cm2), photon flux density (500 μmol m–2 s–1), impeller (7), and relative humidity (25%). Leaves were kept in the leaf chamber in a way that 50% leaf area comes under the sensor zone of the leaf chamber. Leaves of three different plants (3 leaves per plant) were observed for each treatment to measure the leaf gas exchange parameters.
Estimation of photosynthetic pigments
Photosynthetic pigments were extracted in the reaction mixture consisting 7 mL dimethyl sulfoxide and chopped leaf tissue (100 mg) and was heated at 65 ºC for 30 min (Hiscox and Israelstam1979). The absorbance was measured at 453 nm, 645 nm, and 663 nm using a spectrophotometer (Beckman coulter DUR 730 life science UV/Vis). Contents of chlorophyll a, chlorophyll b and carotenoids were calculated following Arnon (1949).
Analysis of plant growth indices
Root washing was done using EDTA method described by Azcue (1996) to avoid any adhering metal on root surface. For this, roots were first soaked in 0.01 M EDTA solution for 3 h and were followed by repeated washing with deionized water. Plants were blotted dry, and used for determination of fresh weight (FW), leaf area index (LAI), root and shoot lengths for assessing the growth indices. For determination of dry weight (DW), plants were uprooted carefully, washed, and dried in oven at 60 ºC.
Estimation of lipid peroxidation and superoxide anion
Fresh leaf tissue (250 mg) was homogenized in 5 mL of 1% TCA and centrifuged at 10,000 g for 10 min at 4 ºC. The reaction mixture containing an aliquot of supernatant (1 mL) and 4 mL of 0.5% thiobarbituric acid reagent prepared in 20% TCA was heated at 95 ºC for 30 min and immediately cooled in ice bath. The specific and non-specific absorbances were recorded at 535 nm and 600 nm, respectively, to calculate the MDA using the extinction coefficient 155 mM−1 cm−1 (Heath and Packer 1968).
The in situ localization of superoxides anion (O2.−) was performed by dipping the leaf tissue in nitroblue tetrazolium (NBT) staining solution [1 mg mL−1 prepared in 100 mM phosphate buffer (pH 7.8)] for 2 h at 37 °C. Afterwards, leaf samples were washed with 100 mM phosphate buffer (pH 7.8) for the removal of excess stain and bleaching of chlorophyll was done in boiling 70% ethanol in a hot water bath at 60 °C. Finally, the blue spots were observed under a light microscope.
Extraction and estimation of enzymeand non-enzyme antioxidants of AsA-GSH cycle
Extraction of AsA-GSH cycle enzymes was done in extraction buffer having ingredients potassium phosphate buffer (50 mM; pH 7.0), 1 mM EDTA and 2% PVP. However, for extraction of APX, 1 mM AsA was added to the extraction buffer. The supernatant was used for assessing the enzyme activities (Noctor et al. 2016). The SOD activity measured in a reaction mixture having ingredients phosphate buffer (50 mM; pH 7.8), EDTA (0.2 mM), L-methionine (9.9 mM), triton- × 100 (0.025%), enzyme extract, and 1 mM riboflavin and absorbance was recorded at 540 nm (Beyer and Fridovich 1987). The reaction mixture for APX activity contained 100 mM chilled phosphate buffer (pH 7.0), 0.1 mM EDTA, 0.6 mM H2O2, 0.5 mM AsA, enzyme extract and the kinetic changes were observed at 290 nm for 180 s. Likewise, the DHAR activity was assessed in a reaction mixture (3 mL) containing potassium phosphate buffer (50 mM; pH 7.0), GSH (2.5 mM), DHA (0.2 mM), EDTA (0.1 mM), enzyme extract and the kinetic changes were observed at 265 nm for 120 s (Nakano and Asada 1981). The MDHAR activity was measured as per the method described by Hossain et al. (1984) in a reaction mixture containing Tris–HCl buffer (50 mM; pH 7.5), NADH (0.2 mM), AsA (2.5 mM), ascorbate oxidase (1 unit), and enzyme extract. The GR activity was assayed in a reaction mixture consisting phosphate buffer (60 mM; pH 7.5), MgCl2 (6.25 mM), GSSG (10 mM), NADPH (0.5 mM), and enzyme extract (Foyer et al. 1997). The oxidation of NADPH was determined in kinetic mode at 340 nm for 120 s.
The non-enzyme antioxidants include reduced and oxidized ascorbate and glutathione. The AsA and DHA were quantified using fresh leaf tissue (0.1 g) homogenized in 10 mL of cold 5% (w/v) TCA and centrifuged at 16,000 g for 10 min at 4 °C. Resulting supernatant was used for quantification of AsA and total AsA, DHA content derived by subtracting AsA from total AsA (Wu et al. 2006). Total glutathione (GSH and GSSG) quantified in fresh leaf tissue (0.2 g) macerated in 2 mL of sulpho salicylic acid (5%) and centrifuged at 12,000 g for 20 min at 4 ºC. The assay mixture for GSH quantification consisted of 1.0 mL supernatant, phosphate buffer (100 mM; pH 7.7), DTNB (0.6 mM); and the reaction mixture for total glutathione composed of DNTB (0.6 mM), NADPH (0.2 mM), potassium phosphate buffer (50 mM; pH 8.0), EDTA (2 mM), glutathione reductase (1 unit), and 0.1 mL of supernatant. The absorbances were recorded at 412 nm. The content of GSSG was derived by subtracting the GSH content from total glutathione (Anderson et al. 1992).
Statistical analysis
The data were statistically analyzed by one-way analysis of variance (ANOVA) using SPSS software (version 21 for Windows 8; IBM Ltd.), and presented as mean ± standard deviation (n = 3, P ≤ 0.05).
Results and discussion
Chromium (VI) uptake and accumulation
Plant roots and shoots exhibited an increased accumulation of Cr(VI) with increase in Cr(VI) concentrations in soil (Table 1). Accumulation of Cr(VI) in roots increased from 0.3 mg Kg−1 DW in control to 3.66 and 12.3 mg Kg−1 DW, respectively in 50 and 100 mg Cr(VI) kg−1 soil. Also, roots accumulated several times more Cr compared to shoots (0.3 and 1.3 mg Kg−1 DW, respectively in 50 and 100 mg Cr(VI) kg−1 soil) which could be attributed to Cr sequestration in the root vacuoles followed by poor translocation of Cr to shoots. Exogenous application of SA decreased the Cr accumulation as compared to their corresponding non-SA treated plants in both roots and shoots. The reductions of 11% and 26% were observed in roots treated with SA in 50 and 100 Cr(VI) kg−1 soil treatments, respectively (Table 1). A similar effect of exogenous SA-mediated decline in Cr concentrations was evident in shoots as well (Table 1), and also supported by plant uptake factor values. It could have been due to SA-mediated regulation of root metal transporters. Recent studies also showed that application of SA lowered the uptake and accumulation of metals such as cadmium in Lemna minor and boron in Brassica napus (Lu et al. 2018; Metwally et al. 2018). The decreasing trends in plant Cr uptake factor (Table 1) can be correlated with the SA-mediated decreasing trends in transpiration rate (Table 2). In SA treated plants, a low accumulation of Cr could be assigned to more than one mechanisms: (i) exclusion of Cr leading to lower Cr concentration in cytoplasm (Lu et al. 2018); (ii) protective role of SA on plasma membrane integrity (Kohli et al. 2017); and (iii) haem oxygenase-1 mediated signaling cascade which results into lowering of metal uptake (Cui et al. 2012).
Table 1.
Chromium (VI) accumulation in roots and shoot of 45-days old tomato grown under different concentrations of Cr(VI) contaminated soil (with and without 0.5 mM SA)
| Treatments | Soil [mg Cr(VI) Kg−1soil] | Root [mg Cr(VI) Kg−1 DW] | Shoot [mg Cr(VI) Kg−1DW] | Plant uptake factor |
|---|---|---|---|---|
| Control | 2.73 ± 0.45e | 0.30 ± 0.04d | ND | ND |
| 0 mg Cr(VI) Kg−1soil + 0.5 mM SA | 1.26 ± 0.11e | 0.24 ± 0.03d | ND | ND |
| 50 mg Cr(VI) Kg−1soil | 43.77 ± 1.42b | 3.66 ± 0.25c | 0.30 ± 0.02b | 0.08 |
| 50 mg Cr(VI) Kg−1soil + 0.5 mM SA | 37.78 ± 0.31c | 3.26 ± 0.20c | 0.14 ± 0.03c | 0.07 |
| 100 mg Cr(VI) Kg−1soil | 65.54 ± 1.94a | 12.30 ± 0.40a | 1.30 ± 0.03a | 0.13 |
| 100mgCr(VI) Kg−1soil + 0.5 mM SA | 34.18 ± 1.69d | 9.10 ± 0.20b | 0.12 ± 0.01c | 0.09 |
ND: Not detectable
Each value is the mean of three replicates ± SD. The superscripts indicate significant difference among the treatments as per the Duncan test (P ≤ 0.05)
Table 2.
Leaf gas exchange parameters in 45-days old tomato grown under different concentrations of Cr(VI) contaminated soil (with and without 0.5 mM SA)
| Treatments | Net photosynthetic rate (µmol CO2 m−2 s−1) | Stomatal conductance (mmol H2O m−2 s−1) | Transpiration rate (mmol H2O m−2 s−1) |
|---|---|---|---|
| Control | 18.27 ± 0.01a | 504.19 ± 0.31b | 0.21 ± 0.01c |
| 0 mg Cr(VI) Kg−1soil + 0.5 mM SA | 17.11 ± 0.01b | 516.69 ± 0.01a | 0.12 ± 0.01d |
| 50mgCr(VI) Kg−1soil | 9.70 ± 0.14e | 466.91 ± 0.91c | 0.20 ± 0.02c |
| 50mgCr(VI) Kg−1soil + 0.5 mM SA | 15.03 ± 0.04c | 406.27 ± 0.53e | 0.19 ± 0.01c |
| 100mgCr(VI) Kg−1soil | 8.00 ± 0.77f | 333.80 ± 1.29f | 0.94 ± 0.05a |
| 100mgCr(VI) Kg−1soil + 0.5 mM SA | 11.28 ± 0.01d | 421.08 ± 0.16d | 0.69 ± 0.01b |
Each value is the mean of three replicates ± SD. The superscripts indicate significant difference among the treatments as per the Duncan test (P ≤ 0.05)
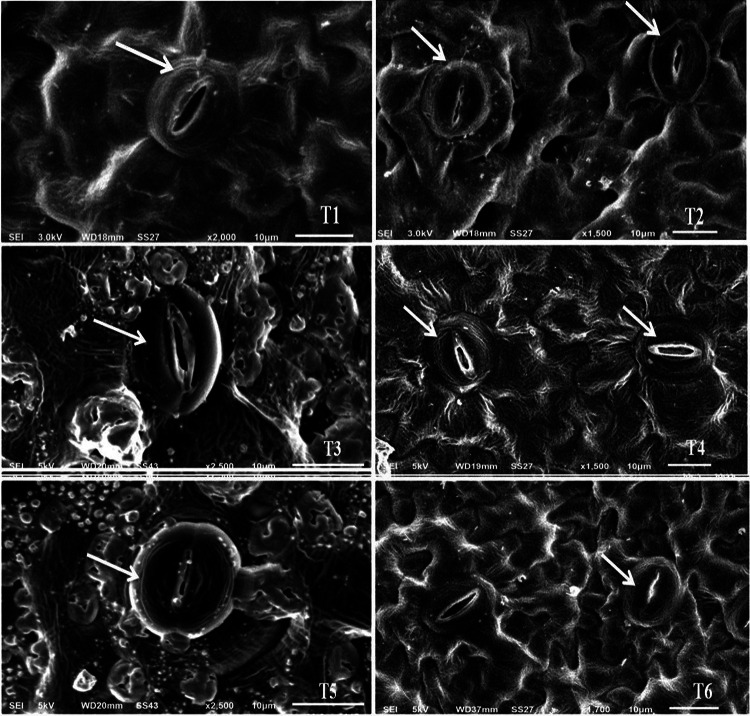
Anomalies in leaf epidermis, leaf gas exchange parameters, and photosynthetic pigments
Chromium (VI) negatively influenced leaf epidermal and stomatal cells that lead to stomatal closure (Fig. 2). The scanning electron micrographs revealed clear damage to the leaf epidermis and stomatal complex, as indicated by abnormal stomata and guard cells (Fig. 2 T3 and T5); while control (T1 and T2) showed intact stomatal aperture and normal epidermal cells. Agnihotri and Seth (2016) have also reported anomalies in guard cells of tomato grown under As(V) stress. However, exogenous application of SA (Fig. 2 T4 and T6) counteracts the negative effects of Cr(VI) on stomatal morphology as indicated by intact stomatal complex and restored leaf epidermal cells structure (Fig. 2). The damaging effect of Cr(VI) on leaf epidermis can also be linked to disturbances observed in gas exchange attributes, however, the ameliorative roles of SA on leaf epidermis damage and stomatal morphology can be linked to SA-mediated reduction in oxidative damage in SA treated plants in present study. In case of leaf gas exchange parameters, results revealed that Cr(VI) decreased net photosynthetic rate and stomatal conductance by 47% and 7%, respectively, in 50 mg Cr(VI) kg−1 soil and by 56% and 34% in 100 mg Cr(VI) kg−1 soil treatment, respectively, compared to control (Table 2). The present observation is in agreement with the report of Ahmad et al. (2020a,b) that showed reduction in gas exhange attributes in Brassica oleracea under Cr stress. The parallel changes of A and GH2O suggested that disturbance in photosynthetic rate was attributed to the stomatal conductance in the present study as indicated by low stomatal conductance at all Cr(VI) concentrations. Furthermore, the impeding effect of Cr(VI) on A is due to the disturbances in electron transport chain and redox change in copper and iron carriers, which in turn, results in inefficient light harvesting capacity of plants (Wakeel et al. 2020). Other reason includes aberration and ultrastructural changes such as poorly developed lamellar system with fewer grana in the chloroplast affecting thylakoid membranes (Gonzalez et al. 2017) and substituting magnesium from chlorophyll (Singh et al. 2016a,b). Additionally, Cr(VI) affects the performance index of photosystem II, thus causing less efficient photosynthesis (Singh et al. 2016a,b). In contrast, moderate Cr concentration showed non-significant effect on transpiration rate, however, at high Cr concentration, it increased significantly (P ≤ 0.05). The altered response of E indicates possible involvement of non-stomatal limitations in controlling photosynthesis in plants exposed to Cr(VI) stress. Results imply that exogenous SA improved photosynthesis at all Cr(VI) concentrations compared to their non-SA counterparts. Salicylic acid is known to cause stomatal closure under stress conditions but it helps in opening the same during non-stress conditions (Acharya et al. 2009), and increases the GH2O and A values. The results suggest the protective influence of SA is linked with decline in E and an increment of photosynthesis, which together enhanced water use efficiency of plant under stress. The inconsistent effect of SA for A vs E can be explained; as photosynthesis is not solely dependent on stomatal opening, but also governed by non-stomatal events like RuBisCO activity, chlorophyll content, light and dark reactions in plant. The improvement in photosynthesis could also be attributed to rapid detoxification of ROS as observed by the low degree of lipid peroxidation and better AsA-GSH cycle in the present study. The ameliorative effects of SA under Cd and Cr stress have been reported by El-Dakak and Hassan (2020) in Zea mays and Zewail et al. (2020) in Basella alba, respectively.
Fig. 2.
Scanning electron micrographs of 45-days old tomato leaves abaxial surface grown under Cr(VI) (T1, T3, and T5 are control, 50 and 100 mg Cr(VI) kg−1 soil, respectively) and Cr (VI) contaminated plants sprayed with SA (0.5 mM) (T2, T4, and T6 are 0 + SA, 50 + SA and 100 mg Cr(VI) kg−1 soil + SA, respectively). Images show structural aberrations in guard cells and their surrounding epidermal cells. Arrows indicates open and intact stomatal aperture, normal shape, and size of guard and epidermal cells in T1 and T2; closed stomata with abnormal epidermal and guard cells in T3 and T5; and completely or partially closed stomata with normal epidermal and intact guard cells in T4 and T6
In case of photosynthetic pigments, chlorophyll a content decreased by 36% in 50 mg Cr(VI) kg−1 soil and 55% in 100 mg Cr(VI) kg−1 soil compared to control. Chlorophyll b and total chlorophyll content decreased by 37% and 38%, respectively, in 50 mg Cr(VI) kg−1 soil and by 54% and 54%, respectively, in 100 mg Cr(VI) kg−1 soil treatment compared with control. However, there was non-significant effect of Cr on ratio of chlorophyll a: chlorophyll b as shown in (Table 3). The reduction in chlorophyll and carotenoids content is due to the inhibitory action of Cr(VI) on chlororophyll synthesis through impaired activities of δ-aminolevulinic acid dehydratase and protochlorophyllide reductase (Ganesh et al. 2008). Singh et al. (2016a,b) also reported alteration of membrane and chloroplast structure in plants under HM stress. The decrease in chlorophyll content by Cr(VI) can also be linked to decrease in absorption of magnesium and nitrogen, which are the crucial structural components of chlorophyll molecule (Singh et al. 2016a,b; Srivastava et al. 2021). However, exogenous application of SA increased chlorophyll a, chlorophyll b, and carotenoids contents by 74%, 67%, and 16%, respectively, in 50 mg Cr(VI) kg−1 soil treatment and by 88%, 91%, and 172%, respectively, in 100 mg Cr(VI) kg−1 soil treatment as compared to control. It is due to its involvement in pigment synthesis, stomatal physiology, and better ROS scavenging. The beneficial effect of SA on total chlorophyll could also be attributed to the maintenance of optimal mineral nutrition under Cd stress as reported by Belkhadi et al. (2010). It is well known that H+-ATPase in plasma membrane plays an important role in the transport of multiple ions (Palmgren and Harper, 1999), and there are investigations indicating that SA could induce H+-ATPase activity (Gordon et al., 2004), which might be responsible for SA increasing absorption of potassium, calcium, magnesium, and iron ions under Cd toxicity. Since these nutrients are active constituents of photosynthetic pigments, SA-mediated positive effect on total chlorophyll can be credited to improved mineral nutrition under stress. Present observations are in accordance with earlier reports confirming an increment in pigment contents by SA application against HM stress in plants (Liu et al. 2016; Moustafa-Farag et al. 2020). Interestingly, a slight decrease in photosynthetic pigments and A in 0 mg Cr(VI) kg−1 soil + SA plants might be attributed to the pro-oxidant nature of SA, where in absence of ROS, SA may itself act as an oxidant (Herrera-Vasquez et al. 2015).
Table 3.
Concentrations of photosynthetic pigments (chlorophyll a, chlorophyll b, total chlorophyll, and carotenoids) and ratios of chlorophyll a:chlorophyll b and total chlorophyll:carotenoids in 45-days old tomato grown under different concentrations of Cr(VI) contaminated soil (with and without 0.5 mM SA)
| Treatments | Chlorophyll a (mg g−1 FW) | Chlorophyll b (mg g−1 FW) | Chlorophyll a:Chlorophyll b | Total Chlorophyll (mg g−1 FW) | Carotenoids (mg g−1 FW) | Total Chlorophyll: Carotenoids |
|---|---|---|---|---|---|---|
| Control | 0.74 ± 0.01b | 0.24 ± 0.04d | 3.13 ± 0.49a | 0.99 ± 0.06b | 1.81 ± 0.15b | 0.54 ± 0.01c |
| 0 mg Cr(VI) Kg−1soil + 0.5 mM SA | 0.53 ± 0.01d | 0.17 ± 0.01b | 3.14 ± 0.15a | 0.70 ± 0.02d | 1.18 ± 0.06d | 0.59 ± 0.01b |
| 50mgCr(VI) Kg−1soil | 0.47 ± 0.03e | 0.15 ± 0.02b | 3.13 ± 0.22a | 0.61 ± 0.04d | 1.26 ± 0.12c | 0.48 ± 0.01d |
| 50 mgCr(VI) Kg−1soil + 0.5 mM SA | 0.82 ± 0.01a | 0.25 ± 0.01a | 3.28 ± 0.09a | 1.07 ± 0.01a | 1.46 ± 0.19c | 0.74 ± 0.09a |
| 100mgCr(VI) Kg−1soil | 0.34 ± 0.01e | 0.11 ± 0.01c | 3.1 ± 0.19a | 0.45 ± 0.01e | 0.79 ± 0.03e | 0.57 ± 0.01b |
| 100 mg Cr(VI) Kg−1soil + 0.5 mM SA | 0.64 ± 0.02c | 0.21 ± 0.01a | 3.04 ± 0.05a | 0.84 ± 0.01c | 2.15 ± 0.16a | 0.39 ± 0.02e |
Each value is the mean of three replicates ± SD. The superscripts indicate significant differences among the treatments as per the Duncan test (P ≤ 0.05)
Analysis of growth indices, lipid peroxidation, superoxides, and AsA-GSH cycle
Chromium (VI) intensified phytotoxicity on growth indices such as plant dry and fresh weight, leaf area index, root length, and shoot length. The results confirmed reduction in dry weight, fresh weight, leaf area index, root length, and shoot length by 52%, 7%, 8%, 42%, and 12%, respectively, at 50 mg Cr(VI) kg−1 soil and further decreased by 65%, 40%, 11%, 60%, and 57%, respectively, at 100 mg Cr(VI) kg−1 soil exposure, compared with control (Table 4). Inhibition of plant growth under Cr(VI) stress is due to the oxidative stress, hindrance in nutrients uptake, dehydration and disturbance of several metabolic activities in the plant (Zewail et al. 2020). Previous studies also reported reduction in growth and yield of Brassica juncea (Mahmud et al. 2017), Helianthus annus (Farid et al. 2019), Cicer arietinum (Singh et al. 2020), and Basella alba (Zewail et al. 2020) under HM stress. However, exogenous application of SA (0.5 mM) alleviated the Cr(VI) induced phytotoxicity and enhanced dry weight, fresh weight, leaf area index, root length, and shoot length by 164%, 41%, 63%, 12%, and 16%, respectively, in 50 mg Cr(VI) kg−1 soil and by 135%, 89%, 43%, 23%, and 34%, respectively, in 100 mg Cr(VI) kg−1 soil treatment compared to control (Table 4). Ali (2017) has observed that SA has improved the dry weight, leaf area, root length, and shoot length in Vigna radiata under aluminium stress. Improved growth and physical parameters by SA is due to its involvement in plant growth, enhanced photosynthesis, and augmented stress tolerance (Farid et al. 2019).
Table 4.
Plant growth indices (dry weight, fresh weight, leaf area index, root length, and shoot length) of 45-day old tomato grown under different concentrations of Cr(VI) contaminated soil (with and without 0.5 mM SA). Each value is the mean of three replicates ± SD
| Treatments | Plant dry weight (g) | Plant fresh weight (g) | Leaf area index | Root length (cm) | Shoot length (cm) |
|---|---|---|---|---|---|
| Control | 0.23 ± 0.02c | 3.86 ± 0.25c,d | 1.05 ± 0.03d | 11.00 ± 1.00a | 35.66 ± 1.15a,b |
| 0mgCr(VI) Kg−1soil + 0.5 mM SA | 0.32 ± 0.03a | 5.58 ± 0.52a | 1.72 ± 0.04a | 10.66 ± 1.15a | 41.33 ± 4.16a |
| 50mgCr(VI) Kg−1soil | 0.11 ± 0.01e | 3.58 ± 0.18d | 0.97 ± 0.01e | 6.40 ± 1.15b | 31.36 ± 4.60b |
| 50 mg Cr(VI) Kg−1soil + 0.5 mM SA | 0.29 ± 0.01b | 5.03 ± 0.21b | 1.58 ± 0.02b | 7.16 ± 0.80b | 36.33 ± 3.05a,b |
| 100mgCr(VI) Kg−1soil | 0.08 ± 0.01f | 2.32 ± 0.28e | 0.93 ± 0.05e | 4.36 ± 1.18c | 15.43 ± 0.93c |
| 100mgCr(VI) Kg−1soil + 0.5 mM SA | 0.19 ± 0.01d | 4.38 ± 0.23c | 1.33 ± 0.04c | 5.36 ± 0.56b | 20.66 ± 7.23c |
The superscripts indicate significant differences among the treatments as per the Duncan test (P ≤ 0.05)
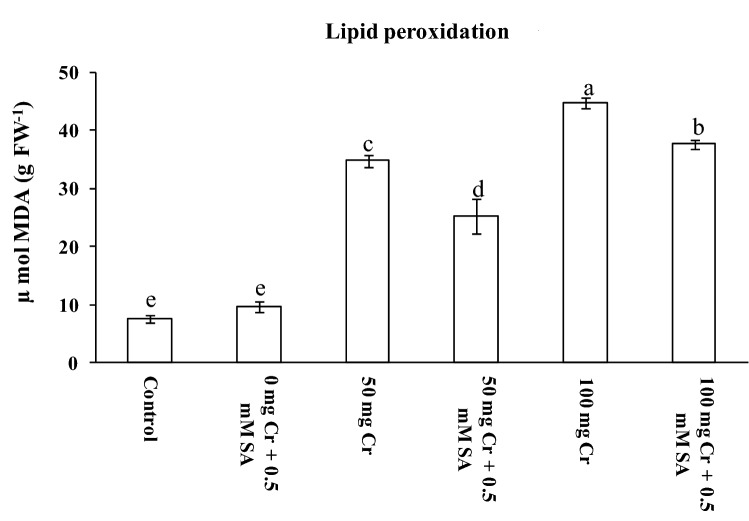
Chromium being a strong oxidant can exist in many oxidative forms and participate in fenton type reactions, disrupts electron transport chain and generates ROS, that consequently initiates lipid peroxidation (Wakeel et al. 2020), as indicated by increased MDA levels in present study (Fig. 3). The MDA content hugely escalated by 354% and 392% in 50 and 100 mg Cr(VI) kg−1 soil treatments, respectively, as compared to control. Exogenous SA has resulted significant (P ≤ 0.05) fall in MDA content by 27% and 16% in 50 and 100 mg Cr(VI) kg−1 soil + SA, respectively, compared to non-SA counterparts (Fig. 3). This is due to its interference in controlling the oxidative damage, endogenous levels of plant hormones, antioxidative defense systems and better scavenging of ROS (Liu et al. 2016; Sharma et al. 2020). The ameliorative effect of SA in mitigating HM induced oxidative stress has also been reported in Sorghum bicolor (Sihag et al. 2019) and Citrullus lanatus (Moustafa-Farag et al. 2020). A slight increase in MDA content in 0 mg Cr(VI) kg−1 soil + SA plants might be due to endogenous redox status of the system. As in case of 0 mg Cr(VI) kg−1 soil treatment, there is no redox imbalance, as a result, SA acted as a pro-oxidant (Herrera-Vasquez et al. 2015), and resulted in slight increase in the MDA content.
Fig. 3.
MDA content in leaves of 45-days old tomato grown under different concentrations of Cr (VI) contaminated soil (with and without 0.5 mM SA). Each value is the mean of three replicates ± SD. The superscripts indicate significant differences among the treatments as per the Duncan test (P ≤ 0.05)
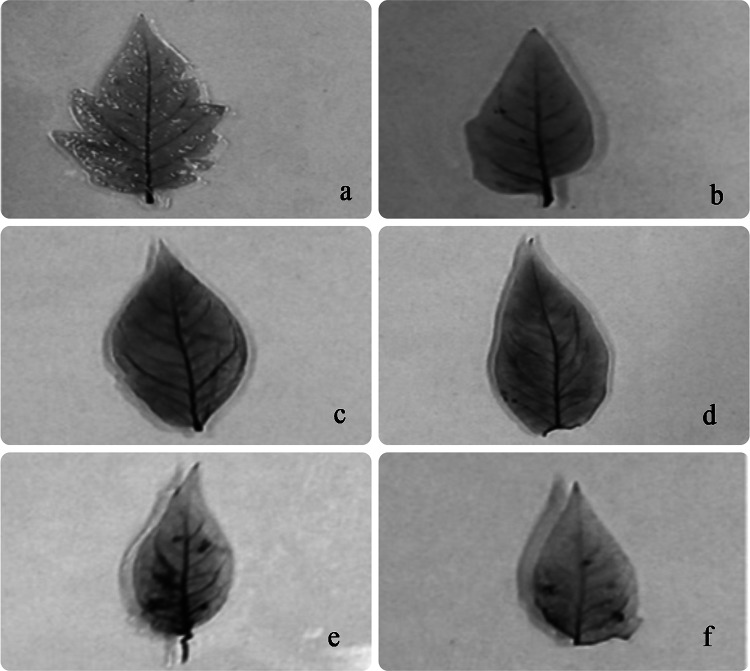
The in situ observations for superoxides anion (O2.−) in the tomato leaves with NBT staining were in agreement with other biochemical parameters as shown in Fig. 4. Deep blue staining was observed in leaves under 50 and 100 mg Cr(VI) kg−1 soil treatments. Chromium stress induces oxidative damage, which causes cellular membrane injury, electrolytic leakage, membrane lipid peroxidation, and DNA damage, thus resulting in cell death (Srivastava et al. 2021). Results of the study revealed that Cr(VI) stress increased the production of O2.− that made membrane lipids more susceptible to peroxidation (confirmed by higher MDA content) and causes cell death. Exogenous application of SA helped in alleviating the superoxide anions (O2.−) accumulation under Cr-toxicity as revealed by the presence of scanty and shallow pattern of blue staining. So, these results clearly indicated that SA plays a significant role in regulating oxidative damage processes under Cr(VI) stress in tomato by the induction of cellular antioxidant machinery which is considered as a vital approach for protection against various abiotic stresses.
Fig. 4.
Histochemical localization of superoxide anions via NBT staining in leaves of 45-days old tomato grown under different concentrations of Cr (VI) contaminated soil (with and without 0.5 mM SA). a Control (0 mg Cr kg−1 soil), b 0 mg Cr kg−1 soil + SA, c 50 mg Cr kg−1 soil, d 50 mg Cr kg−1 soil + SA, e 100 mg Cr kg−1 soil, f 100 mg Cr kg−1 soil + SA
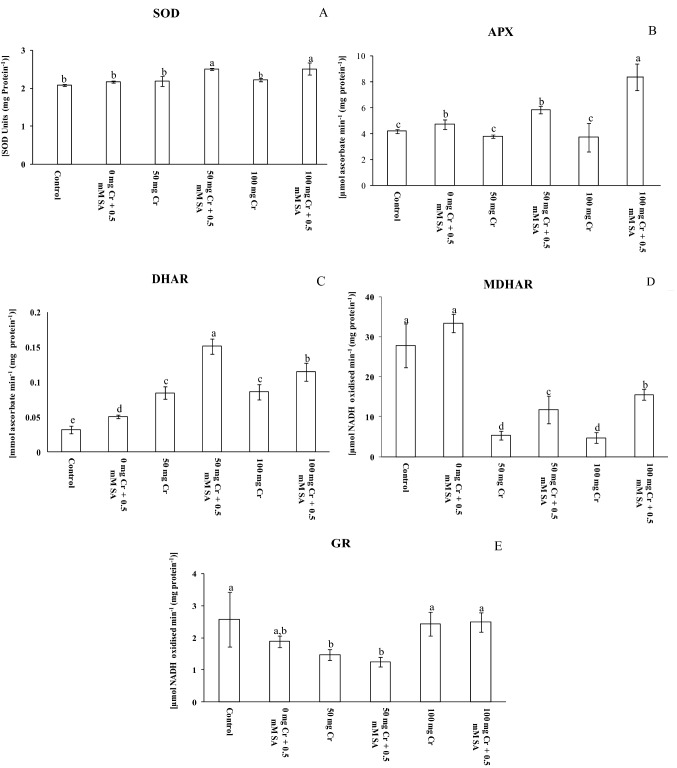
In case of enzyme antioxidants of AsA-GSH cycle, the activity of DHAR increased by 163% in 50 mg Cr(VI) kg−1 soil and by 169%, in 100 mg Cr(VI) kg−1 soil compared to control (Fig. 5 A-E). Contrary to this, a decrease of 10%, 81%, and 43%, respectively, was recorded in activities of APX, MDHAR, and GR at 50 mg Cr(VI) kg−1 soil treatment and more severe effects on these antioxidants were observed at 100 mg Cr(VI) kg−1 soil treatment (Fig. 5 A-E). For non-enzyme components of AsA-GSH cycle, the content of AsA increased by 194% and 331% in 50 and 100 mg Cr(VI) kg−1 soil treatments, respectively, compared with control (Table 5), whereas GSH content increased by 119% and 13% under 50 and 100 mg Cr(VI) kg−1 soil treatments, respectively, compared to control plants (Table 5). The AsA/DHA ratio increased by 26% and 50% in 50 and 100 mg Cr(VI) kg−1 soil, respectively, whereas the GSH/GSSG ratio decreased by 47% and 60% in 50 and 100 mg Cr(VI) kg−1 soil, respectively, compared to control plants (Table 5). The AsA-GSH cycle seems to be functional at moderate exposure of 50 mg Cr(VI) kg−1 soil while it almost failed at extreme toxic dose of 100 mg Cr(VI) kg−1 soil. This can be due to the overproduction of noxious ROS via a fenton-type reaction and sensitivity of all the enzyme and non-enzyme antioxidants to Cr(VI). The present study observed differential response of AsA-GSH cycle components as certain enzymes exhibited higher activity; while others showed limited activity at increased levels of Cr(VI). APX, MDHAR, and DHAR were most sensitive to Cr(VI), while GR significantly increased at elevated cellular Cr(VI) concentrations. Mahmud et al. (2017) also reported that Cr causes decrease in MDHAR and DHAR activity in Brassica juncea. The Cr(VI) stressed plants had a lower AsA level and higher DHA level because of high production of ROS under stress conditions. Reduced AsA content is also involved with the diminished activities of MDHAR (recycle DHA to AsA) under Cr(VI) stress in the present study. Exogenous application of SA significantly (P ≤ 0.05) improved the enzyme activity compared to their Cr(VI) counterparts. The trend is in accordance with improved activities of SOD, APX, and GR in cauliflower and sorghum under Cr stress (Ahmad et al. 2019; Kumar et al. 2019). It has been reported that exogenous application of SA has enhanced the APX, MDHAR, and DHAR activities as well as AsA content, and finally increased the AsA/DHA and GSH/GSSG ratio under Cr(VI) stress. Exogenous SA-mediated increase in AsA and GSH contents is reported earlier in Cucumis melo under cadmium stress (Zhang et al. 2015). The results suggested that SA played a positive role in improving the AsA-GSH cycle and thereby, augmented tolerance against Cr(VI) toxicity in tomato plant.
Fig. 5.
a–e Activities of enzymes of AsA-GSH cycle: a superoxide dismutase (SOD), b ascorbate peroxidase (APX), c dehydroascorbate reductase (DHAR) d monodehydroascorbate reductase (MDHAR), and e glutathione reductase (GR) in leaves of 45-days old tomato grown under different concentrations of Cr (VI) contaminated soil (with and without 0.5 mM SA). Each value is the mean of three replicates ± SD. The superscripts indicate significant differences among the treatments as per the Duncan test (P ≤ 0.05)
Table 5.
Ascorbate and glutathione pool in 45-days old tomato grown under different concentrations of Cr(VI) contaminated soil (with and without 0.5 mM SA). Each value is the mean of three replicates ± SD. The superscripts indicate significant difference among the treatments as per the Duncan test (P ≤ 0.05)
| Treatments | AsA (µg g−1 FW) | DHA (µg g−1 FW) | AsA/DHA | GSH (µg g−1 FW) | GSSG (µg g−1 FW) | GSH/GSSG |
|---|---|---|---|---|---|---|
| Control | 04.44 ± 0.86c | 09.73 ± 1.20c | 0.46 | 1.81 ± 0.62c | 1.71 ± 0.69b,c | 1.30 |
| 0mgCr(VI) Kg−1soil + 0.5 mM SA | 08.72 ± 0.28c | 13.20 ± 0.29c | 0.66 | 3.48 ± 0.45b | 0.59 ± 0.41d | 3.87 |
| 50mgCr(VI) Kg−1soil | 13.06 ± 1.83c | 22.60 ± 2.42b | 0.58 | 3.98 ± 0.16a | 3.31 ± 0.68a | 0.69 |
| 50mgCr(VI) Kg−1soil + 0.5 mM SA | 40.95 ± 0.41a,b | 56.58 ± 1.53a | 0.72 | 4.25 ± 0.57a | 1.04 ± 0.70c,d | 1.25 |
| 100mgCr(VI) Kg−1soil | 19.15 ± 1.55b | 27.71 ± 1.17b | 0.69 | 2.05 ± 0.50c | 0.54 ± 0.645d | 0.52 |
| 100 mg Cr(VI) Kg−1soil + 0.5 mM SA | 53.39 ± 1.58a | 28.74 ± 4.11b | 1.86 | 3.72 ± 0.55a,b | 2.23 ± 0.532b | 2.24 |
AsA ascorbate, DHA dehydroascorbate, GSH glutathione, GSSG glutathione disulphide
Conclusion
The present study concludes that tomato plants exposed to Cr(VI) stress resulted in increased Cr-accumulation in root and shoot and showed inhibition in plant's growth performance as evidenced by low biomass, high degree of oxidative damage proved by escalated MDA and superoxides levels, and inhibition in enzymes of AsA-GSH cycle. Also, notable reduction in photosynthetic pigments and gas exchange parameters was evident in Cr (VI) stressed tomato plants. However, exogenous SA application of 0.5 mM not only promoted plant growth by restricting Cr acquisition and translocation but also positively influenced chlorophyll biosynthesis and improved photosynthesis, lowered the degree of lipid peroxidation and superoxide levels. It also improved the efficiency of AsA-GSH cycle, potentially involved in the quenching of ROS. These observations strengthen the assumption that exogenous SA can act as a crucial signaling molecule in the alleviation of Cr(VI) induced toxicity in tomato plants by augmenting photosynthesis and by strengthening the antioxidant defense system. Further, long-term studies and experiments are warranted to understand as to how exogenous SA performs its signaling and crosstalk with other phytohormones in presence of HMs including Cr stress and helps in the phytoremediation of toxic HMs from contaminated sites. It would help in understanding plant's adaptation ability under various abiotic stress including HM. Also, such type of studies in future will surely help in understanding the various key metabolic and detoxifying mechanisms operating in plants to develop efficient phytoremediation strategies.
Acknowledgements
The authors are thankful to Head, Department of Botany, University of Delhi for providing the research facilities. Mr. Ashish Agnihotri, Ph.D. scholar is deeply apprecited for his crucial contribution in revising this manuscript. The Institution of Eminence (IoE), University of Delhi is greatly acknowledged for providing the assistance in the research.
Authors' contributions
Samta Gupta: Performing, collection and assembly of the data, analysis and interpretation of the data, drafting and writing the manuscript, statistical analysis. Chandra Shekhar Seth: Conception and design of the experiment, critical revision of the article for important intellectual content, final approval of the article, provision of study materials, experimental expertise, obtaining of funding, administrative, technical, or logistic support.
Declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Consent to participate
Yes.
Consent for publication
Yes.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acharya BR, Assmann SM. Hormone interactions in stomatal function. Plant MolBiol. 2009;69:451–462. doi: 10.1007/s11103-008-9427-0. [DOI] [PubMed] [Google Scholar]
- Adagunodo TA, SunmonuLA EME. Heavy metals' data in soils for agricultural activities. Data Brief. 2018;18:1847–1855. doi: 10.1016/j.dib.2018.04.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agnihotri A, Seth CS. Exogenously applied nitrate improves the photosynthetic performance and nitrogen metabolism in Tomato (Solanum lycopersicum L. cv. PusaRohini) under arsenic (V) toxicity. PhysiolMolBiol Plants. 2016;22:341–349. doi: 10.1007/s12298-016-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad R, Ali S, Abid M, Rizwan M, Ali B, Tanveer A, Ahmad I, Azam M, Ghani MA. Glycinebetaine alleviates the chromium toxicity in Brassica oleracea L. by suppressing oxidative stress and modulating the plant morphology and photosynthetic attributes. Environ SciPollut Res. 2020;27:1101–1111. doi: 10.1007/s11356-019-06761-z. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Ali S, Rizwan M, Dawood M, Farid M, Hussain A, Wijaya L, Alyemeni MN, Ahmad P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. 2020;168(2):289–300. doi: 10.1111/ppl.13001. [DOI] [PubMed] [Google Scholar]
- Ahmad R, Ali S, Rizwan M, Dawood M, Farid M, Hussain A, Wijaya L, Alyemeni MN, Ahmad P. Hydrogen sulfide alleviates chromium stress on cauliflower by restricting its uptake and enhancing antioxidative system. Physiol Plant. 2019;168:289–300. doi: 10.1111/ppl.13001. [DOI] [PubMed] [Google Scholar]
- Ali B. Salicylic acid induced antioxidant system enhances the tolerance to aluminium in mung bean (Vigna radiata L. Wilczek) plants. Indian J Plant Physiol. 2017;22:178–189. doi: 10.1007/s40502-017-0292-1. [DOI] [Google Scholar]
- Ali S, Chaudhary A, Rizwan M, Anwar HT, Adrees M, Farid M, Irshad MK, Hayat T, Anjum SA. Alleviation of Cr VI toxicity by glycinebetaine is related to elevated antioxidant enzymes and suppressed Cr VI uptake and oxidative stress in wheat (Triticum aestivum L.) Environ Sci Pollut Res. 2015;22:10669–10678. doi: 10.1007/s11356-015-4193-4. [DOI] [PubMed] [Google Scholar]
- Alloway BJ, editor. Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. Springer Science & Business Media; 2012. [Google Scholar]
- Anderson JV, Chevone BI, Hess JL. Seasonal variation in the antioxidant system of eastern white pine needles evidence for thermal dependence. Plant Physiol. 1992;98:501–508. doi: 10.1104/pp.98.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- APHA, AWWA, WEF (2005) Standard methods for the examination of water and wastewater. 21st edn. Washington, DC
- Arnon DI. Copper enzymes in isolated chloroplasts polyphenol oxidase in Beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azcue JM. Comparison of different cleaning procedures of root material for analysis of trace elements. Int J Environ Anal Chem. 1996;62(2):137–145. doi: 10.1080/03067319608027060. [DOI] [Google Scholar]
- Belkhadi A, Hediji H, Abbes Z, Nouairi I, Barhoumi Z, Zarrouk M, Chaibi W, Djebali W. Effects of exogenous salicylic acid pre-treatment on cadmium toxicity and leaf lipid content in Linum usitatissimum L. Ecotoxicol Environ Saf. 2010;73(5):1004–1011. doi: 10.1016/j.ecoenv.2010.03.009. [DOI] [PubMed] [Google Scholar]
- Beyer WF, Fridovich I. Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem. 1987;161:559–566. doi: 10.1016/0003-2697(87)90489-1. [DOI] [PubMed] [Google Scholar]
- Celma AR, Cuadros F, López-Rodríguez F. Characterisation of industrial tomato by-products from infrared drying process. Food Bioproducts Process. 2009;87(4):282–291. doi: 10.1016/j.fbp.2008.12.003. [DOI] [Google Scholar]
- Chen YY, Tang MY, Wang ST, Wang Q, Zhan WX, Huang G. Evaluation of heavy metal pollution in farmland soil of China based on bibliometrics. Int J Phytoremediation. 2016;47:219–225. [Google Scholar]
- Cui W, Li L, Gao Z, Wu H, Xie Y, Shen W. Haem oxygenase-1 is involved in salicylic acid-induced alleviation of oxidative stress due to cadmium stress in Medicago sativa. J ExpBot. 2012;63:5521–5534. doi: 10.1093/jxb/ers201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Dakak RA, Hassan IA. The alleviative effects of salicylic acid on physiological indices and defense mechanisms of maize (Zea Mays L. Giza 2) stressed with cadmium. Environ Process. 2020;7:873–884. doi: 10.1007/s40710-020-00448-1. [DOI] [Google Scholar]
- Eleftheriou EP, Adamakis IDS, Panteris E, Fatsiou M. Cr VI induced ultrastructural changes and oxidative stress in roots of Arabidopsis thaliana. Int J Mol Sci. 2015;16:15852–15871. doi: 10.3390/ijms160715852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farid M, Ali S, Saeed R, Rizwan M, Bukhari SAH, Abbasi GH, Hussain A, Ali B, Zamir MSI, Ahmad I. Combined application of citric acid and 5-aminolevulinic acid improved biomass, photosynthesis and gas exchange attributes of sunflower (Helianthus annuus L.) grown on chromium contaminated soil. Int J Phytoremediation. 2019;21:760–767. doi: 10.1080/15226514.2018.1556595. [DOI] [PubMed] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM. Hydrogen peroxide-and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol Plant. 1997;100:241–254. doi: 10.1111/j.1399-3054.1997.tb04780.x. [DOI] [Google Scholar]
- Foyer CH, Noctor G. Ascorbate and glutathione: the heart of the redox hub. Plant Physiol. 2011;155:2–18. doi: 10.1104/pp.110.167569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh KS, Baskaran L, Rajasekaran S, Sumathi K, Chidambaram ALA, Sundaramoorthy P. Cr VI stress induced alterations in biochemical and enzyme metabolism in aquatic and terrestrial plants. Colloids Surfaces B. 2008;63:159–163. doi: 10.1016/j.colsurfb.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Gao Y, Xia J. Chromium contamination accident in China: viewing environment policy of China. Environ Sci Technol. 2011;45(20):8605–8606. doi: 10.1021/ES203101F. [DOI] [PubMed] [Google Scholar]
- García-Caparrós P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT. Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev. 2020 doi: 10.1007/s12229-020-09231-1. [DOI] [Google Scholar]
- Global Tomato Industry Report 2020: Trends & Opportunities by Country, Consumption, Production, Price Developments, Imports and Exports (2007–2025).
- Gomes MAD, Hauser-Davis RA, Suzuki MS, Vitória AP. Plant chromium uptake and transport, physiological effects and recent advances in molecular investigations. Ecotoxicol Environ Saf. 2017;140:55–64. doi: 10.1016/j.ecoenv.2017.01.042. [DOI] [PubMed] [Google Scholar]
- Gonzalez A, Mar Gil-Diaz M, Pinilla P, Carmen Lobo M. Impact of Cr and Zn on Growth, Biochemical and Physiological Parameters, and Metal Accumulation by Wheat and Barley Plants. Water Air Soil Pollut. 2017;228:1–17. doi: 10.1007/s11270-017-3507-1. [DOI] [Google Scholar]
- Gordon LK, Minibayeva FV, Rakhmatullina DF, Alyabyev AJ, Ogorodnikova TI, Loseva NL, Valitova YN. Heat production of wheat roots induced by the disruption of proton gradient by salicylic acid. Thermo Chimica Acta. 2004;422(1–2):101–104. doi: 10.1016/j.tca.2004.04.032. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplasts: I Kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Herrera-Vásquez A, Salinas P, Holuigue L. Salicylic acid and reactive oxygen species interplay in the transcriptional control of defense genes expression. FrontPlant Sci. 2015;6:171. doi: 10.3389/fpls.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox JT, Israelstam GF. A method for the extraction of chlorophyll from leaf tissue without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Hossain MA, Nakano Y, Asada K. Monodehydroascorbate reductase in spinach chloroplasts and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:385–395. doi: 10.1093/oxfordjournals.pcp.a076726. [DOI] [Google Scholar]
- Hussain A, Ali S, Rizwan M. Role of zinc-lysine on growth and chromium uptake in rice plants under Cr Stress. J Plant Growth Regul. 2018;37:1413–1422. doi: 10.1007/s00344-018-9831-x. [DOI] [Google Scholar]
- Islam F, Yasmeen T, Arif MS, Riaz M, Shahzad SM, Imran Q, Ali I. Combined ability of chromium (Cr) tolerant plant growth promoting bacteria (PGPB) and salicylic acid (SA) in attenuation of chromium stress in maize plants. Plant Physiol Biochem. 2016;108:456–467. doi: 10.1016/j.plaphy.2016.08.014. [DOI] [PubMed] [Google Scholar]
- Kanagaraj G, Elango L. Chromium and fluoride contamination in groundwater around leather tanning industries in southern India: implications from stable isotopic ratio δ53Cr/δ52Cr, geochemical and geostatistical modelling. Chemosphere. 2019;220:943–953. doi: 10.1016/j.chemosphere.2018.12.105. [DOI] [PubMed] [Google Scholar]
- Kazakis N, Kantiranis N, Kalaitzidou K, Kaprara E, Mitrakas M, Frei R, Vargemezis G, Tsourlos P, Zouboulis A, Filippidis A. Origin of hexavalent chromium in groundwater: the example of Sarigkiol Basin. Northern Greece Sci Total Environ. 2017 doi: 10.1016/j.scitotenv.2017.03.128. [DOI] [PubMed] [Google Scholar]
- Kohli SK, Handa N, Kaur R, Kumar V, Khanna K, Bakshi P, Singh R, Arora S, Kaur R, Bhardwaj R (2017) Role of Salicylic Acid in Heavy Metal Stress Tolerance: Insight into Underlying Mechanism. In: Nazar R, Iqbal N, Khan NA (eds) Salicylic Acid: A Multifaceted Hormone 2017. Springer, Singapore, pp 123–144. 10.1007/978-981-10-6068-7
- Kumar P, Tokas J, Singal HR. Amelioration of chromium VI toxicity in sorghum (Sorghum bicolor L.) using glycine betaine. Sci Rep. 2019;9(1):1–15. doi: 10.1038/s41598-019-52479-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Ding Y, Wang F, Ye Y, Zhu C. Role of SA in resistance to cadmium stress in plants. Plant Cell Rep. 2016;35:719–731. doi: 10.1007/s00299-015-1925-3. [DOI] [PubMed] [Google Scholar]
- Lu Q, Zhang T, Zhang W, Su C, Yang Y, Hu D, Xu Q. Alleviation of cadmium toxicity in Lemna minor by exogenous salicylic acid. Ecotoxicol Environ Saf. 2018;147:500–508. doi: 10.1016/j.ecoenv.2017.09.015. [DOI] [PubMed] [Google Scholar]
- Ma J, Lv C, Xu M, Chen G, Lv C, Gao Z. Photosynthesis performance antioxidant enzymes and ultrastructural analyses of rice seedlings under Cr VI stress. Environ Sci Pollut Res. 2016;23:1768–1778. doi: 10.1007/s11356-015-5439-x. [DOI] [PubMed] [Google Scholar]
- Mahmud JA, Hasanuzzaman M, Nahar K, Rahman A, Hossain MS, Fujita M. γ-aminobutyric acid (GABA) confers chromium stress tolerance in Brassica juncea L. by modulating the antioxidant defense and glyoxalase systems. Ecotoxicology. 2017;26:675–690. doi: 10.1007/s10646-017-1800-9. [DOI] [PubMed] [Google Scholar]
- Metwally AM, Radi AA, El-Shazoly RM, Hamada AM. The role of calcium, silicon and salicylic acid treatment in protection of canola plants against boron toxicity stress. J Plant Res. 2018;22:1–4. doi: 10.1007/s10265-018-1008. [DOI] [PubMed] [Google Scholar]
- Mohamed HI, El-Shazly HH, Badr A. Role of Salicylic Acid in Biotic and Abiotic Stress Tolerance in Plants. In: Lone R, Shuab R, Kamili AN, editors. Plant Phenolics in Sustainable Agriculture. Singapore: Springer; 2020. pp. 533–554. [Google Scholar]
- Moral R, Pedreno JN, Gomez I, Mataix J. Effects of chromium on the nutrient element content and morphology of tomato. J Plant Nutr. 1995;18(4):815–822. doi: 10.1080/01904169509364940. [DOI] [Google Scholar]
- Moustafa-Farag M, Mahmoud HI, Mahmoud A, Elkelish A, Misra AN, Guy KM, Kamran M, Ai S, Zhang M. Salicylic acid stimulates antioxidant defense and osmolyte metabolism to alleviate oxidative stress in watermelons under excess boron. Plants. 2020;9:724. doi: 10.3390/plants9060724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981;22:867–880. doi: 10.1093/oxfordjournals.pcp.a076232. [DOI] [Google Scholar]
- Nguyen TQ, Sesin V, Kisiala A, Emery RN. Phytohormonal roles in plant responses to heavy metal stress: implications for using macrophytes in phytoremediation of aquatic ecosystems. Environ Toxicol Chem. 2021;40:7–22. doi: 10.1002/etc.4909. [DOI] [PubMed] [Google Scholar]
- Noctor G, Mhamdi A, Foyer CH. Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ. 2016;39:1140–1160. doi: 10.1111/pce.12726. [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Harper JF. Pumping with plant P-type ATPases. J Exp Botany. 1999;50:883–893. doi: 10.1093/jxb/50.Special_Issue.883. [DOI] [Google Scholar]
- Rebhi AE, Lounici H, Lahrech MB, Morel JL. Response of Artemisia herba alba to hexavalent chromium pollution under arid and semi-arid conditions. Int J Phytoremediation. 2019;21:224–229. doi: 10.1080/15226514.2018.1524841. [DOI] [PubMed] [Google Scholar]
- Sameena PP, Puthur JT. Cotyledonary leaves effectively shield the true leaves in Ricinuscommunis L. from copper toxicity. Int J Phytoremed. 2021;23:492–504. doi: 10.1080/15226514.2020.1825331. [DOI] [PubMed] [Google Scholar]
- Sarath NG, Puthur JT. Heavy metal pollution assessment in a mangrove ecosystem scheduled as a community reserve. Wetlands Ecol Manage. 2020 doi: 10.1007/s11273-020-09764-7. [DOI] [Google Scholar]
- Shackira AM, Jazeel K, Puthur JT (2021) Phycoremediation and phytoremediation: Promising tools of green remediation. In: Mishra V, Kumar A (eds) Sustainable Environmental Clean-up (pp. 273–293). Elsevier
- Shahid M, Shamshad S, Rafiq M, Khalid S, Bibi I, Niazi NK, Dumat C, Rashid MI. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: a review. Chemosphere. 2017;178:513–533. doi: 10.1016/j.chemosphere.2017.03.074. [DOI] [PubMed] [Google Scholar]
- Sharma A, Kapoor D, Wang J, Shahzad B, Kumar V, Bali AS. Chromium bioaccumulation and its impacts on plants: an overview. Plants. 2020;9:100. doi: 10.3390/plants9010100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Li J, Gu R, Yue L, Wang H, Zhan X, Xing B. Carotenoid and superoxide dismutase are the most effective antioxidants participating in ROS scavenging in phenanthrene accumulated wheat leaf. Chemosphere. 2018;197:513–525. doi: 10.1016/j.chemosphere.2018.01.036. [DOI] [PubMed] [Google Scholar]
- Sihag S, Brar B, Joshi UN. Salicylic acid induces amelioration of chromium toxicity and affects antioxidant enzyme activity in Sorghum bicolor L. Int J Phytoremed. 2019;21:293–304. doi: 10.1080/15226514.2018.1524827. [DOI] [PubMed] [Google Scholar]
- Singh S, Parihar P, Singh R, Singh VP, Prasad SM. Heavy metal tolerance in plants: role of transcriptomics, proteomics, metabolomics, and ionomics. Front Plant Sci. 2016;6:1143. doi: 10.3389/fpls.2015.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh VP, Kumar J, Singh M, Singh S, Prasad SM, Dwivedi R, Singh MPVVB. Role of salicylic acid-seed priming in the regulation of chromium(VI) and UV-B toxicity in maize seedlings. Plant Growth Regul. 2016;78:79–91. doi: 10.1007/s10725-015-0076-4. [DOI] [Google Scholar]
- Singh D, Sharma NL, Singh CK, Sarkar SK, Singh I, Dotaniya ML. Effect of chromium (VI) toxicity on morpho-physiological characteristics, yield, and yield components of two chickpea (Cicer arietinum L.) varieties. PLoS ONE. 2020;15(12):e0243032. doi: 10.1371/journal.pone.0243032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava D, Tiwari M, Dutta P, Singh P, Chawda K, Kumari M, Chakrabarty D. Chromium stress in plants: toxicity. Tolerance Phytoremed Sustain. 2021;13(9):4629. [Google Scholar]
- Sumalan RM, Ciulca SI, Poiana MA, Moigradean D, Radulov I, Negrea M, Sumalan RL. The antioxidant profile evaluation of some tomato Landraces with soil salinity tolerance correlated with high nutraceuticaland functional value. Agronomy. 2020;10:500. doi: 10.3390/agronomy10040500. [DOI] [Google Scholar]
- Tan S, Ke Z, Chai D, Miao Y, Luo K, Li W. Lycopene, polyphenols and antioxidant activities of three characteristic tomato cultivars subjected to two drying methods. Food Chem. 2021;338:128062. doi: 10.1016/j.foodchem.2020.128062. [DOI] [PubMed] [Google Scholar]
- Tumolo M, Ancona V, De Paola D, Losacco D, Campanale C, Massarelli C, Uricchio VF. Chromium pollution in european water, sources, health risk, and remediation strategies: an overview. Int J Env Res Pub He. 2020;17:5438. doi: 10.3390/ijerph17155438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakeel A, Xu M, Gan Y. Chromium-induced reactive oxygen species accumulation by altering the enzymatic antioxidant system and associated cytotoxic, genotoxic, ultrastructural, and photosynthetic changes in plants. Int J MolSci. 2020;21:728. doi: 10.3390/ijms21030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu QS, Zou YN, Xia RX. Effects of water stress and arbuscular mycorrhizal fungi on reactive oxygen metabolism and antioxidant production by citrus (Citrus tangerine L.) roots. Eur J Soil Biol. 2006;42:166–172. doi: 10.1016/j.ejsobi.2005.12.006. [DOI] [Google Scholar]
- Yuan L, Shu S, Sun J, Guo S, Tezuka T. Effects of 24-epibrassinolide on the photosynthetic characteristics, antioxidant system and chloroplast ultrastructure in Cucumis sativus L under Ca(NO3)2 stress. Photosynth Res. 2012;112:205–214. doi: 10.1007/s11120-012-9774-1. [DOI] [PubMed] [Google Scholar]
- Zewail RM, El-DesoukeyHS IKR. Chromium stress alleviation by salicylic acid in Malabar spinach (Basella alba) J Plant Nutri. 2020;43:1268–1285. doi: 10.1080/01904167.2020.1727504. [DOI] [Google Scholar]
- Zhang Y, Xu S, Yang S, Chen Y. Salicylic acid alleviates cadmium-induced inhibition of growth and photosynthesis through up-regulating antioxidant defense system in two melon cultivars (Cucumis melo L.) Protoplasma. 2015;252:911–924. doi: 10.1007/s00709-014-0732-y. [DOI] [PubMed] [Google Scholar]
- Ziegler RG, Vogt TM. Tomatoes, lycopene, and risk of prostate cancer. Pharmaceutical Biol. 2002;40:59–69. doi: 10.1076/phbi.40.7.59.9177. [DOI] [Google Scholar]