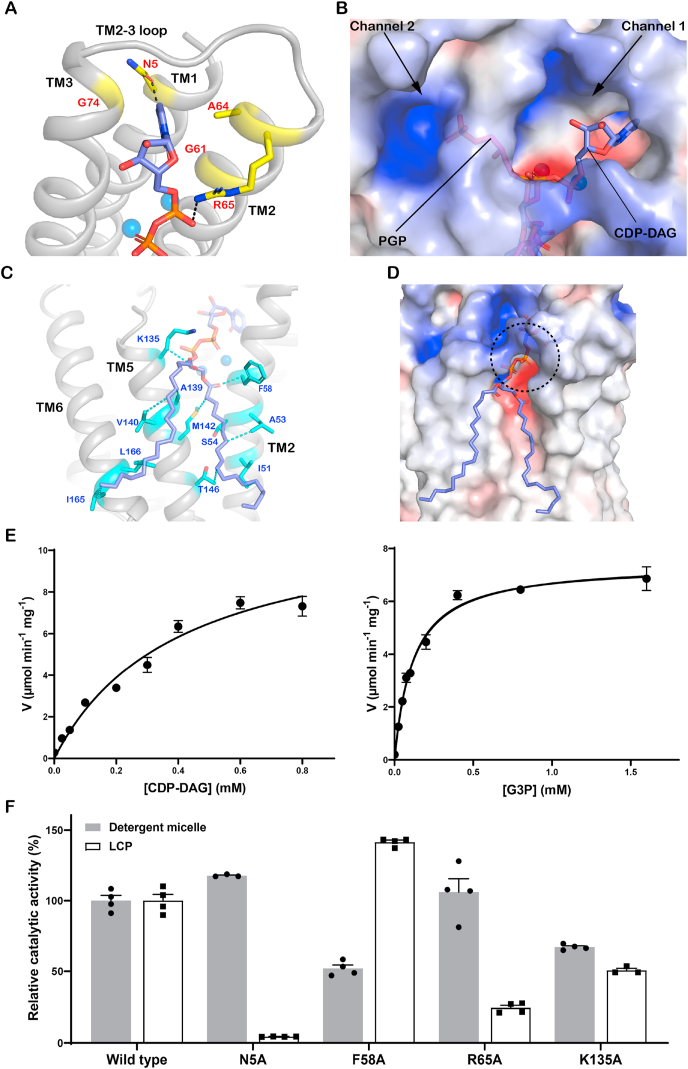
Fig. 3.
The binding site of CDP-DAG in SaPgsA.
(A) Interactions of the CDP moiety of CDP-DAG with SaPgsA. The black dashed lines indicate hydrogen bonds and salt bridges at bond lengths of 2.4 Å or 3.1 Å. (B) Putative CMP release channel (Channel 1) and G3P entrance channel (Channel 2). The PGP molecule from the SaPgsA−PGP complex structure is superposed onto the SaPgsA−CDP-DAG complex structure to indicate the putative G3P location. (C) Interaction of the fatty acyl chains of CDP-DAG with adjacent amino acid residues in SaPgsA. The relevant residues are highlighted in cyan. The cyan dashed lines indicate hydrophobic and van der Waals interactions at distance of 3.1–4.0 Å. (D) The putative entrance for CDP-DAG head group as indicated by the black dashed circle. (E) Substrate-dependent kinetic measurement of SaPgsA activity in detergent micelle. The data points are plotted as mean value ± standard error (SEM as indicated by the error bars. n=3 or 4). For those data points with small errors, the error bars are buried within the symbols. (F) The relative catalytic activity of four alanine mutants related to the CDP-DAG−binding site. The error bars indicate ± SEM with n = 3 or 4. The solid grey bars represent the activity data measured with WT and mutant enzymes solubilized in detergent, whereas the white bars represent the data measured with enzymes embedded in LCP. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)