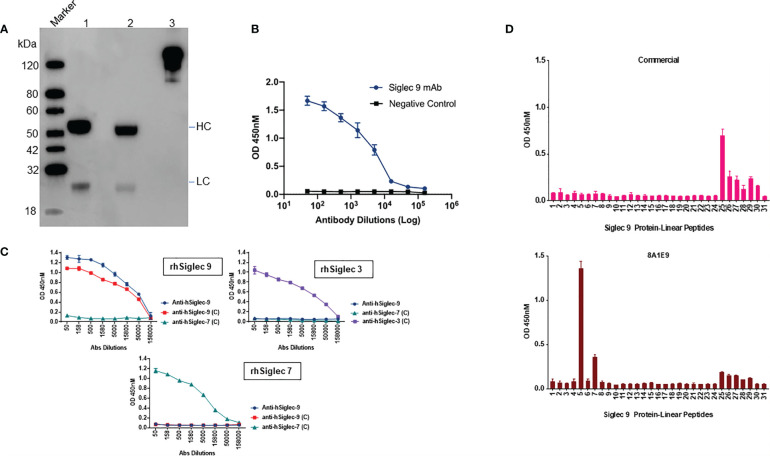
Figure 2.
Characterization of recombinantly expressed human Siglec-9. (A) Western blot analysis of recombinantly expressed anti-Siglec-9. Recombinant antibodies are expressed monoclonal antibodies that are generated in vitro using synthetic genes. The purified recombinant antibody was analyzed by SDS-PAGE, Western blot analysis to determine the molecular weight and purity under non-reducing (lane 3) and reducing conditions (lane 2) alongside isotype control human IgG1, Kappa antibody (lane 1). Reducing and non-reducing loading buffer was added to protein sample respectively, and the final concentration of protein was 0.5 mg/ml. HC, Heavy Chain; LC, Light Chain. (B) Siglec-9 antigen binding of recombinantly expressed Siglec-9 antibodies was measured by ELISA. Assay plates were coated with recombinant Siglec-9, and recombinantly expressed mAbs were used. The binding was determined by ELISA. (C) Recombinantly expressed monoclonal antibodies were tested by ELISA to determine the binding specificity of anti-hSiglec-9. Assay plates were coated with recombinant human Siglec 3, 7, and 9 and then probed with recombinantly expressed anti-hSiglec-9 as well commercial antibodies (labeled as C) against each other to test immune cross reactivity. Anti-hSiglec-9 showed binding specificity to only recombinant hSiglec-9 and did not exhibit any binding to hSiglec-3 or hSiglec-7. (D) To determine potential epitope(s) regions for anti-Siglec-9 antibody binding, an indirect ELISA was performed. Peptide-based ELISA was performed using 100 μL/well of peptide at 2 μg/mL. ELISA plates were coated with 20mer peptides of human Siglec 9 protein as indicated, and recombinant anti-Siglec 9 and commercial antibodies samples were diluted 1:50 and analysed by ELISA as indicated in the Materials and Methods. Data are mean ± SDs for three wells (representative of two independent experiments).