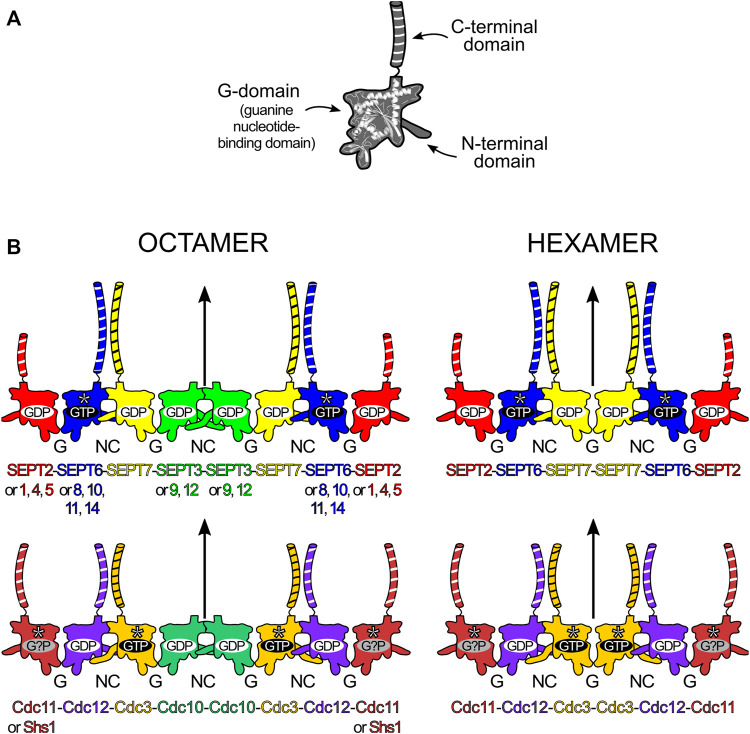
FIGURE 1.
Structural elements and core particle composition of septin filaments. (A) The septin structure is divided into three main domains: the N-terminal domain, the guanine nucleotide-binding domain (or G-domain) and the C-terminal domain (depicted with black and white stripes). (B) Octameric (left) and hexameric (right) core particles in human (top) and baker’s yeast (Saccharomyces cerevisiae, bottom). In human septins, replacements according to “Kinoshita’s rule” are depicted on the octamer. Straight arrows represent the twofold rotational symmetry (diad) axis at the center of the core particle. The yeast hexameric oligomer presented is that formed in the absence of Cdc10 (Cdc10-less oligomer). SEPT6-group members (blue), Cdc3 and Cdc11 are catalytically inactive and do not hydrolyse GTP (asterisks). Cdc11 seems to bind only cytosolic GDP (although speculatively) and Shs1 can also cap octamers, replacing Cdc11. The long N-domains in SEPT3 and Cdc3 are also represented.