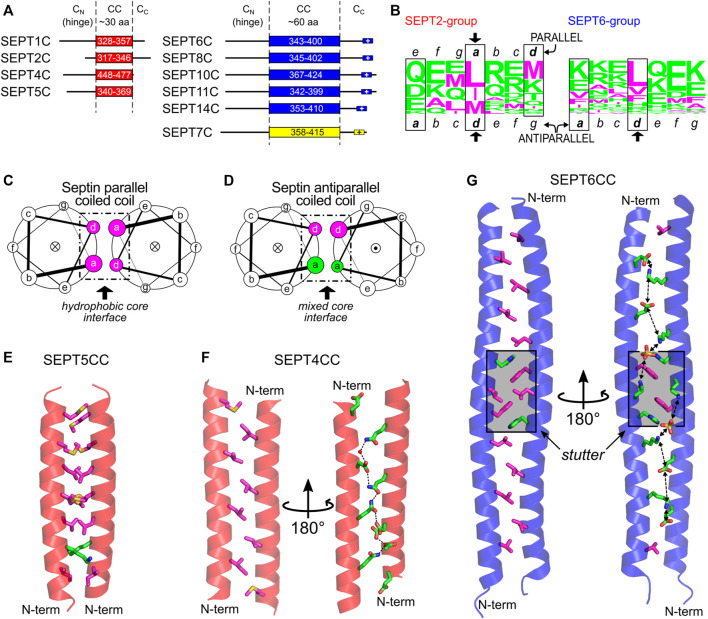
FIGURE 10.
The C-domain and the septin coiled coils. (A) C-domain elements in SEPT2- (red), SEPT6- (blue) and SEPT7-group members (yellow) as reported by Leonardo et al. (Leonardo et al., 2021). The following Uniprot entries were used for residue numbering: SEPT1, Q8WYJ6; SEPT2, Q15019; SEPT4, O43236; SEPT5, Q99719; SEPT6, Q14141-2; SEPT7, Q16181; SEPT8, Q92599-2; SEPT11, Q9NVA2; SEPT14, Q6ZU15. (B) CC heptad motifs in members of human SEPT2 (left) and SEPT6 group (right) represented using a residue frequency sequence logo. Identified core positions are highlighted in boxes and register assignment is shown as seen in the X-ray structures. General helical wheel schemes for (C) parallel and (D) antiparallel human septin coiled coils, showing the different chemical composition of residues in the core (a and d positions). The dot (●) or cross (×) inside the circles represent the helix direction, outward- and inward-pointing, respectively. (E) The parallel septin coiled coil of SEPT5 from the SEPT2 group (PDB:6WCU) showing mainly hydrophobic a and d side chain residues as sticks. (F) The antiparallel septin coiled coil of SEPT4 from the SEPT2 group (PDB:6WB3) showing the two sides of the interface (d-side, left; a-side, right) and the chain of hydrogen bonds seen on the hydrophilic side. (G) Antiparallel septin coiled coil of SEPT6 (PDB:6WBP) showing the two sides of the interface (as in panel (F), but including the aromatic residue of the stutter, in both views) and the chain of favorable salt bridges seen on the hydrophilic a side. The stutter region is highlighted in grey. Throughout the figure, magenta and green colors represent hydrophobic and hydrophilic residues, respectively.