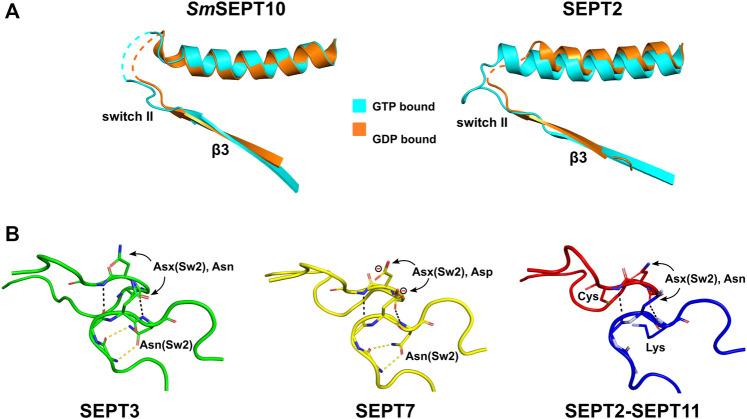
FIGURE 8.
Switch II diversity at the G-interface. (A) β-slippage of the β3-strand in SmSEPT10 and SEPT2. After GTP hydrolysis, the β3-strand slides in the direction of the G-interface, which prevents the formation of the correct contacts between the two copies of switch II (the β-bridge). PDB:4KVA (left; GTP, cyan), PDB:4KV9 (left; GDP, orange), PDB:3FTQ (right; GMPPNP, cyan), PDB:2QNR (right; GDP, orange). (B) The β-bridge (characterized by the hydrogen bonds shown as black dashed lines) in homotypic and heterotypic G-interfaces. Asx(Sw2) residues, conserved in septins, are paired across the interface. In SEPT7, this position is an aspartic acid (formal charge displayed). An asparagine residue, Asn(Sw2), conserved in the SEPT3 and SEPT7 groups, forms hydrogen bonds (yellow dashed lines) with the main chain, helping to stabilize the β-bridge. A lysine substitutes this aspargine in the SEPT6 group and cysteines appear in this position in SEPT1 and SEPT2, suggesting a new mechanism to stabilize the β-bridge in these cases. SEPT3, PDB:4Z54; SEPT7, PDB: 6N0B; SEPT2-11, PDB:6UPQ.