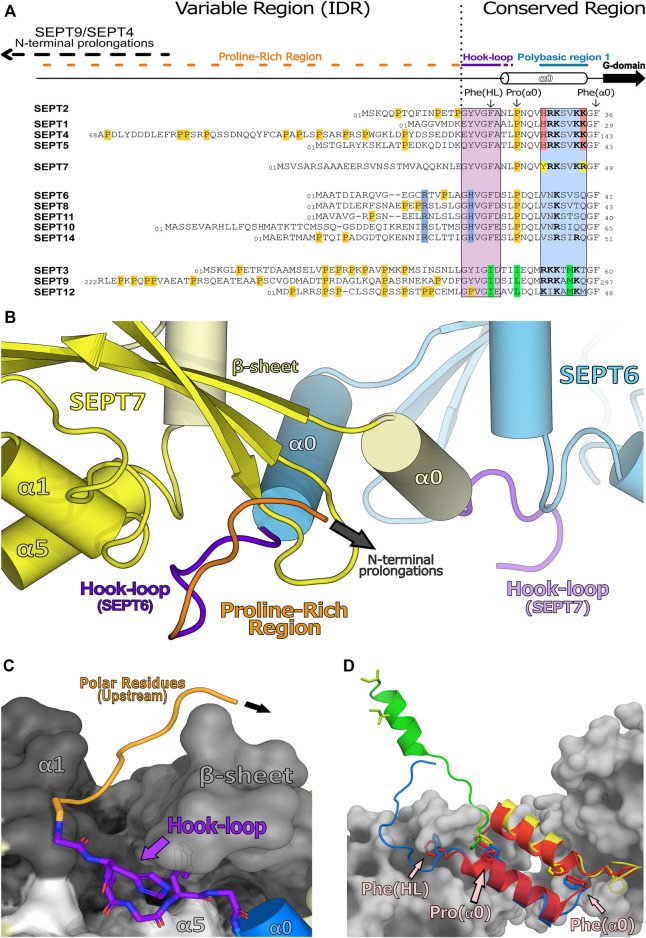
FIGURE 9.
Human septin N-domains and their structured regions. (A) Alignment of all human N-terminal sequences showing the unstructured region (variable region or intrinsically disordered region, IDR) and the structured region (conserved region). This alignment also highlights the conserved residues within a septin group (also known as characteristic residues, in their respective colors) as well as proline residues (in orange). (B) Domain-swapped “hook-loop” highlighting the unstructured proline region (orange) and the hook-loop (purple) (PDB:7M6J). (C) Elements of SEPT7 (in greyscale) which accommodate the hook-loop of SEPT6 into a cleft. Residues with small side chains from the “hook-loop” are important for these interactions. (D) Helix α0 and its orientations within available septin structures (SEPT3 in green, PDB:4Z54; SEPT6 in blue-SEPT7 in yellow, PDB:7M6J; SEPT2 in red, PDB:2QA5). Also highlighted, as sticks, are residues which are essential for the NC-interface and/or the conformation of α0 (Phe(HL), Phe(α0) and Pro(α0); indicated in only one chain for clarity). These phenylalanines act as anchors and are conserved in SEPT2, SEPT6 and SEPT7 groups but are mutated to isoleucines in the SEPT3 group which may be related to additional α0 mobility in the latter.