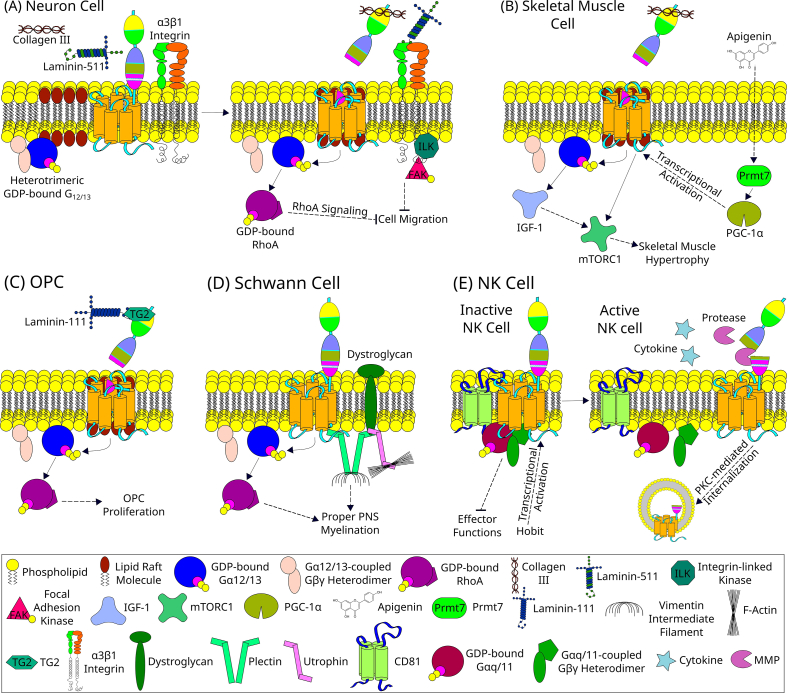
Fig. 2.
GPR56 signaling in physiological processes. (A) In a neuron cell, collagen III acts as a ligand of GPR56 [28]. During the inactive state, GPR56 is found in detergent-soluble non-lipid raft regions of the plasma membrane [102]. However, in the presence of collagen III, the NTF of GPR56 binds to the collagen III which subsequently leads to removal of NTF from the CTF [103]. The shedding of NTF exposes the tethered Stachel agonistic peptide within the CTF [33]. The removal of NTF is also followed by the translocation of CTF to lipid rafts [103]. The tethered Stachel sequence then binds to and induces the conformational changes in CTF to activate the Gα12/13 protein [28]. Upon the GDP-GTP exchange, this heterotrimeric G-protein complex dissociates into Gα12/13 and Gβγ subunits. The Gα12/13 subunit then activates RhoA, which activates various downstream signaling molecules to finally result in the inhibition of migration of the neuron [28]. Some observations suggest a possible interaction of GPR56 with integrin α3β1 [43]. Laminin-511 is known to bind to the integrin α3β1. However, the details of this interaction of GPR56 with integrin remains elusive. (B) In the skeletal muscle cells, resistance exercises and apigenin, a natural flavone found in many edible plants, induces muscle hypertrophy [48,49]. Apigenin enters the cell and activates Prmt7, which then directly activates PGC-1α [49]. The PGC-1α is a transcriptional coactivator which enhances GPR56 and collagen III expression, thereby finally inducing muscle hypertrophy via the IGF-1-mTORC1 pathway [48,49]. (C) In an oligodendrocyte progenitor cell (OPC), the tripartite signaling complex formed of TG2 released by microglia, laminin-111 from ECM, and GPR56 on its cell surface induce the myelin formation and repair in CNS by promoting OPC proliferation and inhibiting its premature differentiation to oligodendrocytes via the Gα12/13-RhoA signaling pathway [47,96]. (D) In a Schwann cell, GPR56 in association with other transmembrane and cytoskeletal linker proteins, like dystroglycan and plectin, induces proper PNS myelination by cytoskeletal remodeling via the RhoA pathway and physical interaction with plectin [29]. (E) Hobit is the primary driver of GPR56 expression in human cytotoxic NK cells [54]. GPR56 in association with CD81 inhibits the effector functions of cytotoxic NK cells during inactive state [19,54]. However, NK cell activation leads to the cleavage of a portion of GPR56 ECR and induces PKC-mediated internalization of the GPR56 receptor, thereby removing its inhibitory effector functions [54]. The solid lines represent direct interaction, whereas the dotted lines indicate indirect pathways with potential additional intermediate(s).