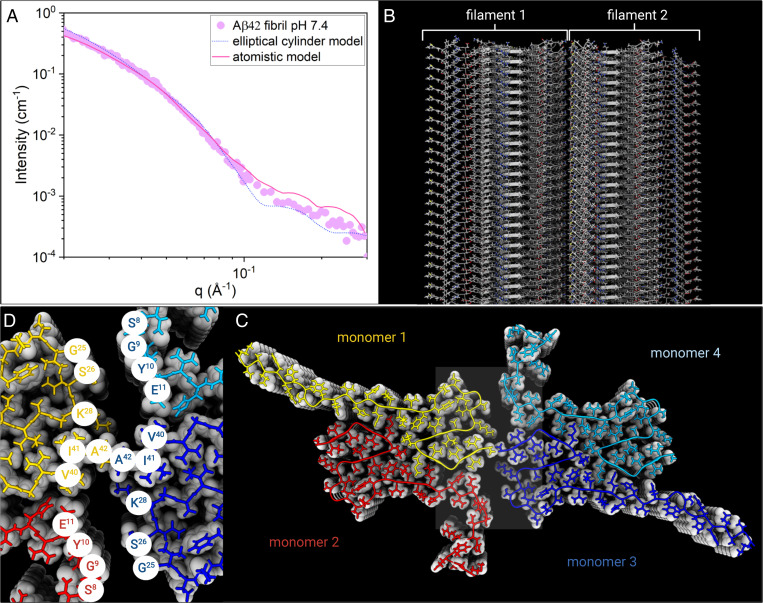
Fig. 3.
(A) Experimental SAXS profile for Aβ42 fibril in 20 mM sodium phosphate buffer at pH = 7.4 (purple circles), plotted together with the best fit of the atomistic model by Pepsi-SAXS (pink line) and the elliptical cylinder model calculation (blue dots). (B) Illustration of the atomistic model structure of the fibrils made by two filaments. (C) A fibril Top view illustrates the fibril cross-section made by tetramer units (monomers 1 to 4, color coded) that form β-sheets stacked along the fibril axis. (D) Zoom of Aβ42 fibril core showing the amino acid residues of contact between filament 1 and 2.