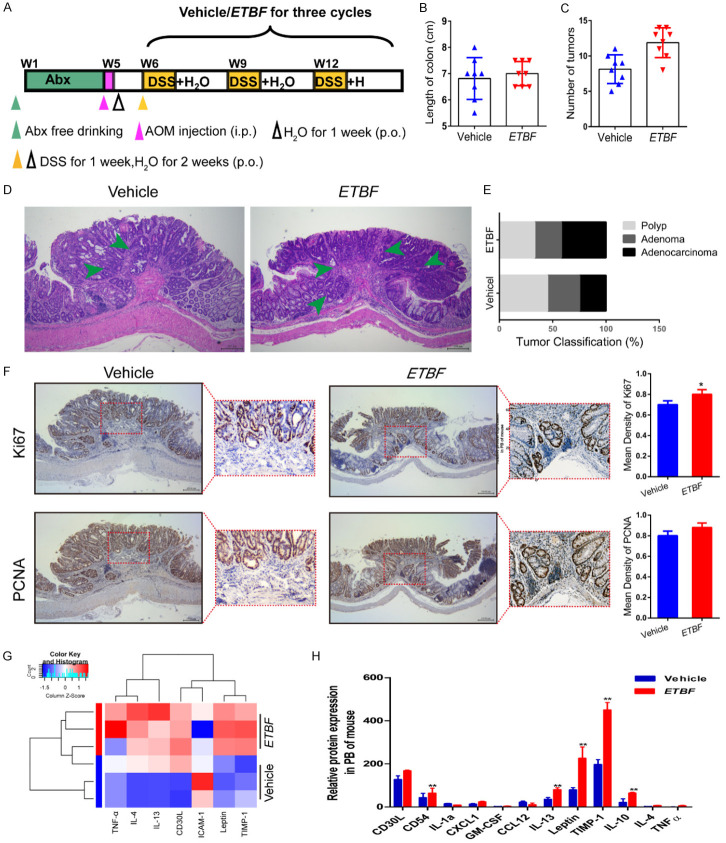
Figure 3.
ETBF infection enhances colonic tumorigenesis and reduces the inflammatory response in the AOM/DSS mouse model. (A) Experimental design indicating the timing of the intragastric administration and organization of groups. Mice were treated with Abx from W1 (age at week 4) to W4, then injected with AOM (12.5 mg/kg, i.p.) and provided drinking water for 1 week, and three cycles of DSS and drinking water for 3 weeks as described in the Methods section. During the treatment, ETBF and Vehicle were orally administered at a dosage of 108 CFU/mice. (B, C) Effects of ETBF on colon length (B) and the number of tumors (C) in the GF/AOM/DSS mouse model. (D) Typical adenomatous intestinal polyp with an early invasion of neoplastic glands into the muscular layers was often observed in ETBF infected GF/AOM/DSS mice. This typical regressive intestinal cancer morphology is observed throughout the intestine in the mice. Blue arrows indicated adenocarcinoma cells. Magnification bars, 100 μM. (E) Histology evaluation of colon tumors: quantitatively represented as polyp, adenoma, and adenocarcinoma. (F) Immunohistochemical staining using an antibody against Ki67 and PCNA in the vehicle group and ETBF infection group. Magnification bars, 100 μM. Data are presented as the means ± SD of 5 regions per slice with Welch’s correction, two-tailed t-test. **P<0.05, vs. Vehicle. (G) The difference between the two groups in inflammatory cytokines as assessed by cytokine antibody array. (H) The mRNA expression levels of inflammatory factors, such as IL-12, TNF-α, and IL-10 in the PB of mice in different groups. The data are presented as the mean ± SD from at least three experiments. **P<0.01 vs. Vehicle.