ABSTRACT.
Transmission risk of Chagas disease has been associated with human-vector contacts and triatomines colonizing dwellings, but alternative scenarios, independent of domestic colonization, are poorly documented. In the present work, we estimated the frequency of human blood meals in triatomines from domicile, peridomicile, and sylvatic environments in two endemic regions in Ecuador. Blood meal origins were identified by sequencing a cytb gene fragment. Human blood meals were detected in 42% of the triatomines among 416 analyzed, including 48% of sylvatic triatomines (both adults and nymphs). In triatomines from domicile and peridomicile, Trypanosoma cruzi infection rate was > 20%, and reached 48% in sylvatic triatomines. Human is a common source of blood for triatomines whether they live in or near dwellings in both regions, and the high rate of T. cruzi infection represents an important risk of transmission of Chagas disease. Consequently, control strategies should also take into account possible nondomestic transmission.
Triatomines are obligatory hematophagous insects that transmit the parasite Trypanosoma cruzi causing Chagas disease (CD) in humans. Although triatomine populations that adapt and colonize human dwellings are more likely to contribute to human infection with T. cruzi, the presence of human blood in sylvatic triatomines has challenged the idea of human transmission restricted to indoors.1–3
In coastal and southern Ecuador, seroprevalence data indicates active transmission of CD.4 Eight species have been identified as vectors in domiciliary and peridomiciliary environments (Panstrongylus chinai, Panstrongylus rufotuberculatus, Panstrongylus howardi, Rhodnius ecuadoriensis, Triatoma carrioni, Triatoma dimidiata, and Triatoma dispar).5–8 All these species, except T. carrioni and T. dimidiata, have been also collected in sylvatic environments.9,10
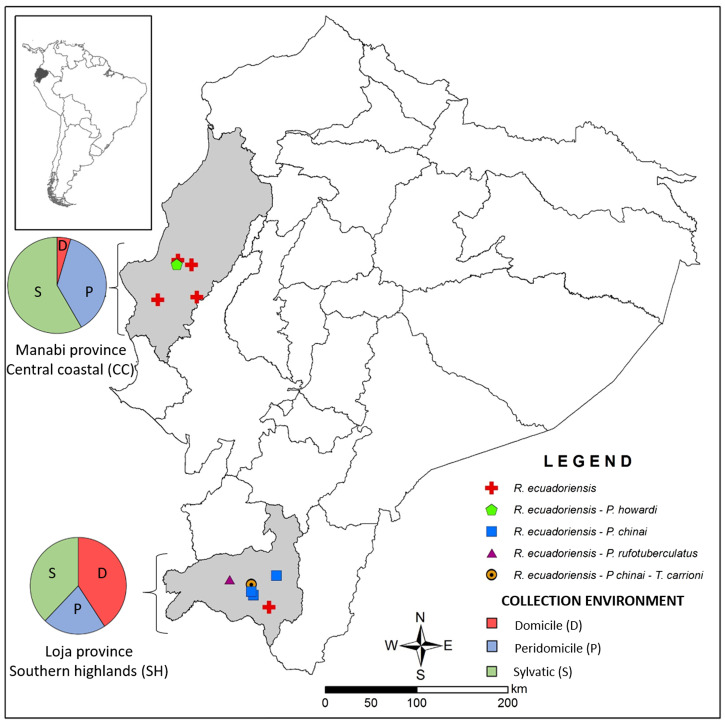
After the collection of triatomines in Manabí province (central coastal region [CC]) and Loja province (southern highland region [SH]) in Ecuador, numerous human blood meals, that are currently analyzed, have been previously identified, and we proposed new scenarios of vectorial transmission11 (Figure 1).
Figure 1. Geographical origin of the 170 triatomines with human blood meals. The triatomines were from domestic, peridomestic, and sylvatic environments in five localities in central costal region (CC) and six localities in southern highlands (SH) regions in Ecuador. This figure appears in color at www.ajtmh.org.
Briefly, of 507 analyzed triatomines, 416 blood meals (one per individual) were identified, 40.9% were human blood (N = 170), and the 337 remaining ones were from other mammals than human (27%), birds (31%), amphibians (0.5%), and reptiles (0.5%).11 The blood meals were detected using DNA isolated from the intestinal contents by the sequencing of a cytb gene fragment amplified according to previously reported conditions12 and biosafety protocols were applied to avoid contamination by handling. Forward and reverse sequences of each sample were edited and corrected with MEGA version 6 (https://www.megasoftware.net/). Consensus sequences were compared with the ones of cytb in GenBank and those with an identity and cover ≥ 95% were retained. For the other samples, no sequences were obtained (N = 53) or they were unreadable with several ambiguities that can be explained by mixture of sequences (N = 7), or the sequence did not match with 95% identity with any sequence of Genbank (N = 31).
The 170 corrected sequences identified as fragments of human cytb (99% were from forward and reverse strands) varied from 121 base pair (bp) to 304 bp. To detect diversity among them, an alignment of 164 bp long was performed with 94 of these sequences, the other sequences being smaller, using MEGA version 6 and DnaSP version 5 software (http://www.ub.edu/dnasp/). Shorter alignment would decrease genetic information and possibly observed diversity. Two variable nucleotide positions were found, and four different haplotypes were identified. The most common haplotype gathers 62 sequences (H2), the second one 21 sequences (H1); both were present in domestic, peridomestic, and sylvatic environments (see below the description of the environments). In addition, 8 (H3) and 3 (H4) sequences represented the two less frequent haplotypes, H3 was present in peridomestic and sylvatic habitats, and H4 only in the sylvatic one.
Human blood was detected in all five species of collected triatomines in both geographic areas (38.1% in SH and 45.9% in CC) and in all environments: domicile (45/83, 54.2%), peridomicile (47/171, 27.5%), and sylvatic (78/162, 48.1%) (Figure 1). Domiciliary triatomines were collected indoors, in structures such as bed, crack of walls, cardboard boxes, posters, and others; peridomiciliary ones were collected in different structures (chicken nests, piles of wood, roof tiles, bricks, shed, corral, and others) located in the peridomicile defined as the space around the house, delimited by a fence or not, where pile of materials and domestic animal shelters are placed, and used by the inhabitants.13 Finally, triatomines collected outside domestic and peridomestic areas were considered to be collected in sylvatic environment of different level of anthropization from crops to forest; collections were mostly done in rodent nests (squirrel nests found in trees).
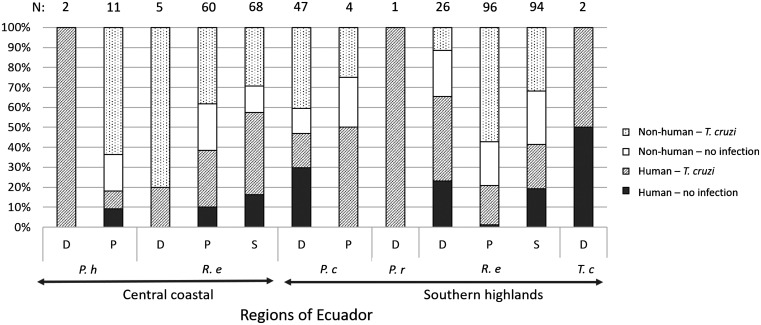
Figure 2 summarized the distribution of the human blood meals per geographic areas, vector species, and environments. In CC, in P. howardi, the two individuals from domicile and 18.2% from the peridomicile (N = 11) presented human blood meals. In SH, the few tested blood meals from P. rufotuberculatus (N = 1) and T. carrioni (N = 2) collected in domiciles, had also human blood. For P. chinai, similar proportion of human blood meal was found in bugs collected in domiciles and peridomiciles (46.8% [N = 47], and 50% [N = 4], respectively). For R. ecuadoriensis, the principal species widely distributed in both regions, human blood meals were detected from all environments. In domiciles frequencies were 20% (N = 5) in CC and 65.4% (N = 26) in SH; however, no significant differences were found (Fisher’s Exact Test, P = 0.083). In peridomiciles, where this species is very abundant, significant differences were found between CC (38.3%, N = 60) and SH (20.8%, N = 96) (Fisher’s Exact Test, P = 0.015). Unexpectedly, in sylvatic environments, human blood meals were found in high proportions, and significant differences were found between specimens from SH (41.5%, N = 94) and CC (57.4%, N = 68) (Fisher’s Exact Test, P = 0.033). Only R. ecuadoriensis were collected in the sylvatic environment (N = 162) and human blood was detected in 70% of the collection points. These collection points were classified according to their distance to the nearest house in three categories, < 100 m (N = 19), 101–200 m (N = 25) and > 200 m (N = 26). The percentage of collection point with at least one triatomines with human blood meal range from 68% to 73% and no significant difference of frequencies was observed between the three categories of distance (Fisher’s Exact Test, P = 0.89). Additionally, of the 49 houses where human blood meals was detected, 42.9% had their closeness house infested (presence of at least one triatomine), while in the group of collection point where no human blood was detected only 19.1% were close to an infested house, but the difference between the two groups was not significant (Fisher’s Exact Test, P = 0.064).
Figure 2. Histogram of the geographical, species, and environmental distribution of identified human blood meals. Percentage of triatomines with human (black area) and nonhuman (stripped area) blood meals, per Ecuadorian regions, vector species (P. h: P. howardi, P. c: P. chinai, P. r: P. rufotuberculatus, R. e: R. ecuadoriensis, and T. c: T. carrioni) and collection environments (D: domicile, P: peridomicile, and S: sylvatic). The number above the columns indicates the number of triatomines.
For the 170 triatomines with human blood meals, Table 1 summarizes the numbers of infected specimens with T. cruzi according to their stage, species, and place of capture (region, habitat). A total of 65.9% were positive for T. cruzi. The infection reached 69.8% in R. ecuadoriensis (N = 139), and 41.7% for P. chinai (N = 24); some specimens of all other species (P. howardi, P. rufotuberculatus, and T. carrioni) were found infected, but sample sizes are too small for the evaluation of their percentage. Overall, the analyzed samples, infection rates were also important in triatomines collected in domiciles (53.3%, 24/45), in peridomiciles (83%, 39/47), and in sylvatic (62.8%, 49/78) environments. Both adults and nymphs presented a high T. cruzi infection rates (> 70% in CC and > 57% in SH).
Table 1.
T. cruzi infection rates of the 170 triatomines where human blood meal was detected
| Triatomine species/environment | CC | SH | Total | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adults | Nymphs | Total CC | Adults | Nymphs | Total SH | |||||||||
| n | Tc (%) | n | Tc (%) | n | Tc (%) | n | Tc (%) | n | Tc (%) | n | Tc (%) | n | Tc (%) | |
| P. chinai | – | – | – | – | – | – | 6 | 1 (16.7) | 18 | 9 (50.0) | 24 | 10 (41.7) | 24 | 10 (41.7) |
| Domicile | – | – | – | – | – | – | 6 | 1 (16.7) | 16 | 7 (43.8) | 22 | 8 (36.4) | 22 | 8 (36.4) |
| Peridomicile | – | – | – | – | – | – | – | 2 | 2 (100.0) | 2 | 2 (100.0) | 2 | 2 (100.0) | |
| P. howardi | 3 | 2 (66.7) | 1 | 1 (100.0) | 4 | 3 (75.0) | – | – | – | 4 | 3 (75.0) | |||
| Domicile | 2 | 2 (100.0) | – | – | 2 | 2 (100.0) | – | – | – | 2 | 2 (100.0) | |||
| Peridomicile | 1 | 0 (0.0) | 1 | 1 (100.0) | 2 | 1 (50.0) | – | – | – | 2 | 1 (50.0) | |||
| P. rufotuberculatus | – | – | – | – | – | – | 1 | 1 (100.0) | – | 1 | 1 (100.0) | 1 | 1 (100.0) | |
| Domicile | – | – | – | – | – | – | 1 | 1 (100.0) | – | 1 | 1 (100.0) | 1 | 1 (100.0) | |
| R. ecuadoriensis | 30 | 23 (76.7) | 33 | 23 (69.7) | 63 | 46 (73.0) | 44 | 31 (70.5) | 32 | 20 (62.5) | 76 | 51 (67.1) | 139 | 97 (69.8) |
| Domicile | – | 1 | 1 (100.0) | 1 | 1 (100.0) | 13 | 7 (53.8) | 4 | 4 (100.0) | 17 | 11 (64.7) | 18 | 12 (66.7) | |
| Peridomicile | 15 | 11 (73.3) | 8 | 6 (75.0) | 23 | 17 (73.9) | 12 | 12 (100.0) | 8 | 7 (87.5) | 20 | 19 (95.0) | 43 | 36 (83.7) |
| Sylvatic | 15 | 12 (80.0) | 24 | 16 (66.7) | 39 | 28 (71.8) | 19 | 12 (63.2) | 20 | 9 (45.0) | 39 | 21 (53.8) | 78 | 49 (62.8) |
| T. carrioni | – | – | – | – | – | – | – | 2 | 1 (50.0) | 2 | 1 (50.0) | 2 | 1 (50.0) | |
| Domicile | – | – | – | – | – | – | – | 2 | 1 (50.0) | 2 | 1 (50.0) | 2 | 1 (50.0) | |
| Total | 33 | 25 (75.8) | 34 | 24 (70.6) | 67 | 49 (73.1) | 51 | 33 (64.7) | 52 | 30 (57.7) | 103 | 63 (61.2) | 170 | 112 (65.9) |
| Domicile | 2 | 2 (100.0) | 1 | 1 (100.0) | 3 | 3 (100.0) | 20 | 9 (45.0) | 22 | 12 (54.5) | 42 | 21 (50.0) | 45 | 24 (53.3) |
| Peridomicile | 16 | 11 (68.8) | 9 | 7 (77.8) | 25 | 18 (72.0) | 12 | 12 (100.0) | 10 | 9 (90.0) | 22 | 21 (95.5) | 47 | 39 (83.0) |
| Sylvatic | 15 | 12 (80.0) | 24 | 16 (66.7) | 39 | 28 (71.8) | 19 | 12 (63.2) | 20 | 9 (45.0) | 39 | 21 (53.8) | 78 | 49 (62.8) |
CC = central coastal region; SH = southern highland region; Tc (%) = number of samples positive for T. cruzi (infection rate). Bold values represent the total samples and T. cruzi infection rates by species.
Overall, the high percentage of triatomines with human blood meal (40.7%) and their high infection rate with T. cruzi, indicated that human is a more important feeding source than previously thought, so it exists a high risk of parasite transmission to humans. In the domestic environment, triatomines with human blood meals are expected because it is well known that the transmission of CD arises when colonization or occasional entering of triatomines occurs.14 In peridomestic and sylvatic environments, the presence of human blood meals poses the question on how these triatomines (adults and nymphs) are getting in contact with humans. As mentioned above, triatomines were mostly collected in chicken nest in the peridomicile, and in squirrel nest in the sylvatic environment. We suggest three scenarios. The first one is the dispersion of triatomines from peridomestic and sylvatic environments to indoors. Indeed, the presence of open spaces between the walls and roof facilitates the access of insects from outside to indoors.15 In addition, laying hens are frequently installed against the wall of the house or very close (< 50 m distance) allowing triatomine dispersion by flying or walking to the house. Interestingly, in Manabí province (CC region) part of the hot spot density of R. ecuadoriensis in sylvatic environment was previously reported nearby houses (< 50 m).9 Moreover, dispersal of adult triatomines could be explained by light attraction.16 The second scenario is triatomine–human contact during outdoor activities of inhabitants. Although, the contact of humans with sylvatic triatomines has been associated to an expansion for human settlements2 or by accidental contact with humans (e.g., during camping),1 the risk of transmission might be also related with activities in the field (e.g., farming, sheltering cattle, etc.).3 Nevertheless, anthropological studies are needed to determine if people have contact with triatomines during outdoor activities and which of those activities could constitute a major risk of transmission. The third scenario is related to the cleptohematophagy behavior of triatomines that has been previously reported under experimental conditions.17 In this study, human blood meals were detected in R. ecuadoriensis nymphs collected too far from any house to assume that they had walked to the house to feed and then returned to their refuge. In this case, feeding on adult congeners gorged with human blood would be a rational explanation of human blood presence in nymphs. However, further experiments need to be carried out to assess this behavior in R. ecuadoriensis.
This study reinforces the importance of including other variables such as the influence of seasonality in triatomine behavior and human activities that could increase human–vector interactions. Considering local conditions is key to evaluate and propose integral control and prevention measures. Although spraying has an effect on decreasing domestic triatomine populations, it might not be the main strategy to prevent the transmission of CD in Ecuador. In these areas, previous studies reported 5.7% in CC and 3.6% seroprevalence rates4; however, the high frequency of human blood meals observed in this study suggests numerous contacts between inhabitants and vectors, and a possible underestimation of CD cases. This is in agreement with previous statements of, among others, the need of more research for a better understanding of triatomine transmission scenarios.18
ACKNOWLEDGMENTS
We want to thank the personnel from the National Chagas Control Program of the Ecuadorian Ministry of Health, Alejandra Zurita, Amber McDonald, and Santiago Cadena for the technical assistance in the field and laboratory.
REFERENCES
- 1. Stevens L Dorn PL Hobson J de la Rua NM Lucero DE Klotz JH Schmidt JO Klotz SA , 2012. Vector blood meals and Chagas disease transmission potential, United States. Emerg Infect Dis 18: 646–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Waleckx E Suarez J Richards B Dorn PL , 2014. Triatoma sanguisuga blood meals and potential for Chagas disease, Louisiana, USA. Emerg Infect Dis 20: 2141–2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buitrago NL Bosseno MF Waleckx E Bremond P Vidaurre P Zoveda F Breniere SF , 2013. Risk of transmission of Trypanosoma cruzi by wild Triatoma infestans (Hemiptera: Reduviidae) in Bolivia supported by the detection of human blood meals. Infect Genet Evol 19: 141–144. [DOI] [PubMed] [Google Scholar]
- 4. Black CL Ocana-Mayorga S Riner DK Costales JA Lascano MS Arcos-Teran L Preisser JS Seed JR Grijalva MJ , 2009. Seroprevalence of Trypanosoma cruzi in rural Ecuador and clustering of seropositivity within households. Am J Trop Med Hyg 81: 1035–1040. [DOI] [PubMed] [Google Scholar]
- 5. Grijalva MJ Villacis AG Moncayo AL Ocana-Mayorga S Yumiseva CA Baus EG , 2017. Distribution of triatomine species in domestic and peridomestic environments in central coastal Ecuador. PLoS Negl Trop Dis 11: e0005970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Grijalva MJ Villacis AG Ocana-Mayorga S Yumiseva CA Moncayo AL Baus EG , 2015. Comprehensive survey of domiciliary triatomine species capable of transmitting Chagas disease in southern Ecuador. PLoS Negl Trop Dis 9: e0004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guevara A Moreira J Criollo H Vivero S Racines M Cevallos V Prandi R Caicedo C Robinzon F Anselmi M , 2014. First description of Trypanosoma cruzi human infection in Esmeraldas province, Ecuador. Parasit Vectors 7: 358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Quinde-Calderon L Rios-Quituizaca P Solorzano L Dumonteil E , 2016. Ten years (2004–2014) of Chagas disease surveillance and vector control in Ecuador: successes and challenges. Trop Med Int Health 21: 84–92. [DOI] [PubMed] [Google Scholar]
- 9. Grijalva MJ Teran D Dangles O , 2014. Dynamics of sylvatic Chagas disease vectors in coastal Ecuador is driven by changes in land cover. PLoS Negl Trop Dis 8: e2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suarez-Davalos V Dangles O Villacis AG Grijalva MJ , 2010. Microdistribution of sylvatic triatomine populations in central-coastal Ecuador. J Med Entomol 47: 80–88. [DOI] [PubMed] [Google Scholar]
- 11. Ocana-Mayorga S Bustillos JJ Villacis AG Pinto CM Breniere SF Grijalva MJ , 2021. Triatomine feeding profiles and Trypanosoma cruzi infection, implications in domestic and sylvatic transmission cycles in Ecuador. Pathogens 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Buitrago R Depickere S Bosseno MF Patzi ES Waleckx E Salas R Aliaga C Breniere SF , 2012. Combination of cytochrome b heteroduplex-assay and sequencing for identification of triatomine blood meals. Infect Genet Evol 12: 21–27. [DOI] [PubMed] [Google Scholar]
- 13. Walter A Lozano-Kasten F Bosseno MF Ruvalcaba EG Gutierrez MS Luna CE Baunaure F Phelinas P Magallon-Gastelum E Breniere SF , 2007. Peridomicilary habitat and risk factors for Triatoma infestation in a rural community of the Mexican occident. Am J Trop Med Hyg 76: 508–515. [PubMed] [Google Scholar]
- 14. Breniere SF Villacis AG Aznar C , 2017. Vector transmission: how it works, what transmits, where it occurs. Telleria J, Tibayrenc M, eds. American Trypanosomiasis Chagas Disease: One Hundred Years of Research. pp. 497–515.
- 15. Black CL Ocana S Riner D Costales JA Lascano MS Davila S Arcos-Teran L Seed JR Grijalva MJ , 2007. Household risk factors for Trypanosoma cruzi seropositivity in two geographic regions of Ecuador. J Parasitol 93: 12–16. [DOI] [PubMed] [Google Scholar]
- 16. Pacheco-Tucuch FS Ramirez-Sierra MJ Gourbiere S Dumonteil E , 2012. Public street lights increase house infestation by the Chagas disease vector Triatoma dimidiata. PLoS One 7: e36207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schaub GA Boker CA Jensen C Reduth D , 1989. Cannibalism and coprophagy are modes of transmission of Blastocrithidia triatomae (Trypanosomatidae) between triatomines. J Protozool 36: 171–175. [DOI] [PubMed] [Google Scholar]
- 18. Dumonteil E Herrera C Martini L Grijalva MJ Guevara AG Costales JA Aguilar HM Breniere SF Waleckx E , 2016. Chagas disease has not been controlled in Ecuador. PLoS One 11: e0158145. [DOI] [PMC free article] [PubMed] [Google Scholar]