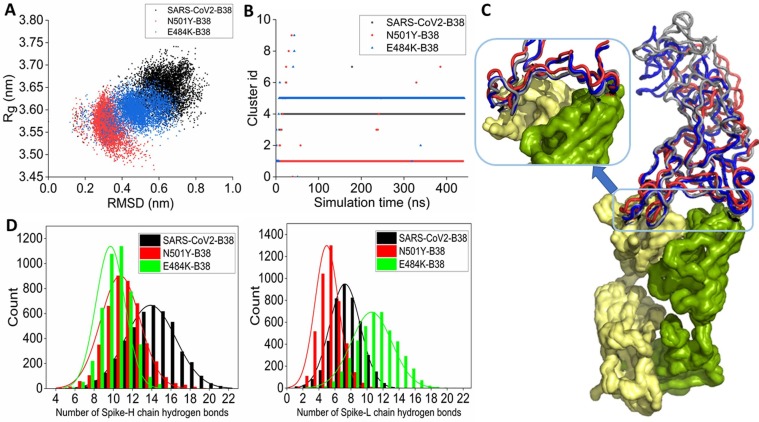
Fig. 5.
(A) 2-D scatter representation of the conformational ensemble of wild-type RBD-B38 (black), N501Y RBD-B38 (red), and E484K RBD-B38 (blue) complexes obtained from the equilibrium simulation in RMSD and Rg space. (B) Time-evolution of conformational clusters of wild-type (black), N501Y (red), and E484K (blue) RBD complexed with B38. (C) The alignment of the average complex structure from the most populated clusters for the three systems is shown. Wild-type, N501Y, and E484K RBD are colored gray, red, and blue. B38 is represented in surface mode. (D) The distribution of the number of hydrogen bonds between the wild-type and mutant RBDs and the H-chain (left) and L-chain (right) of the antibody. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)