Figure 5.
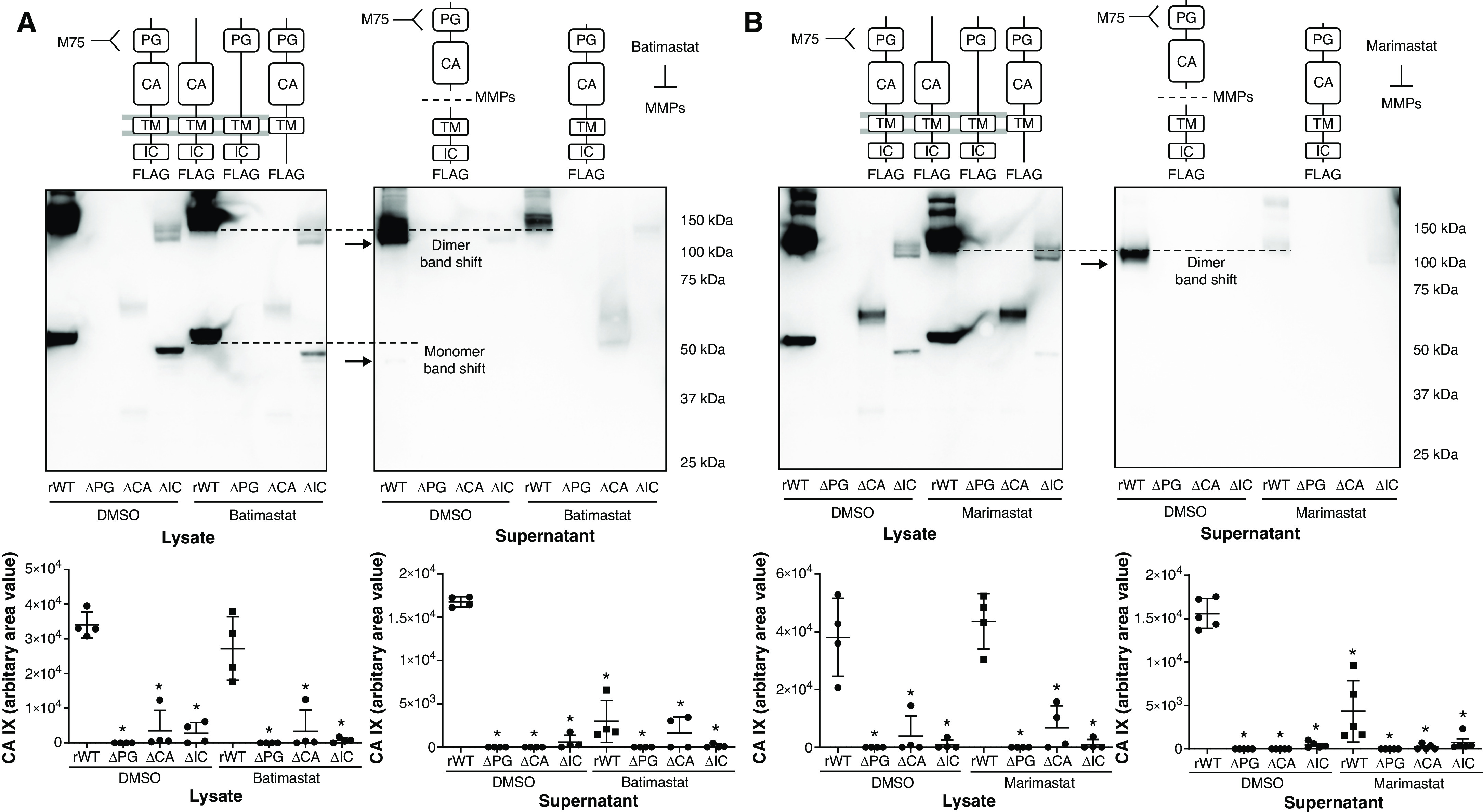
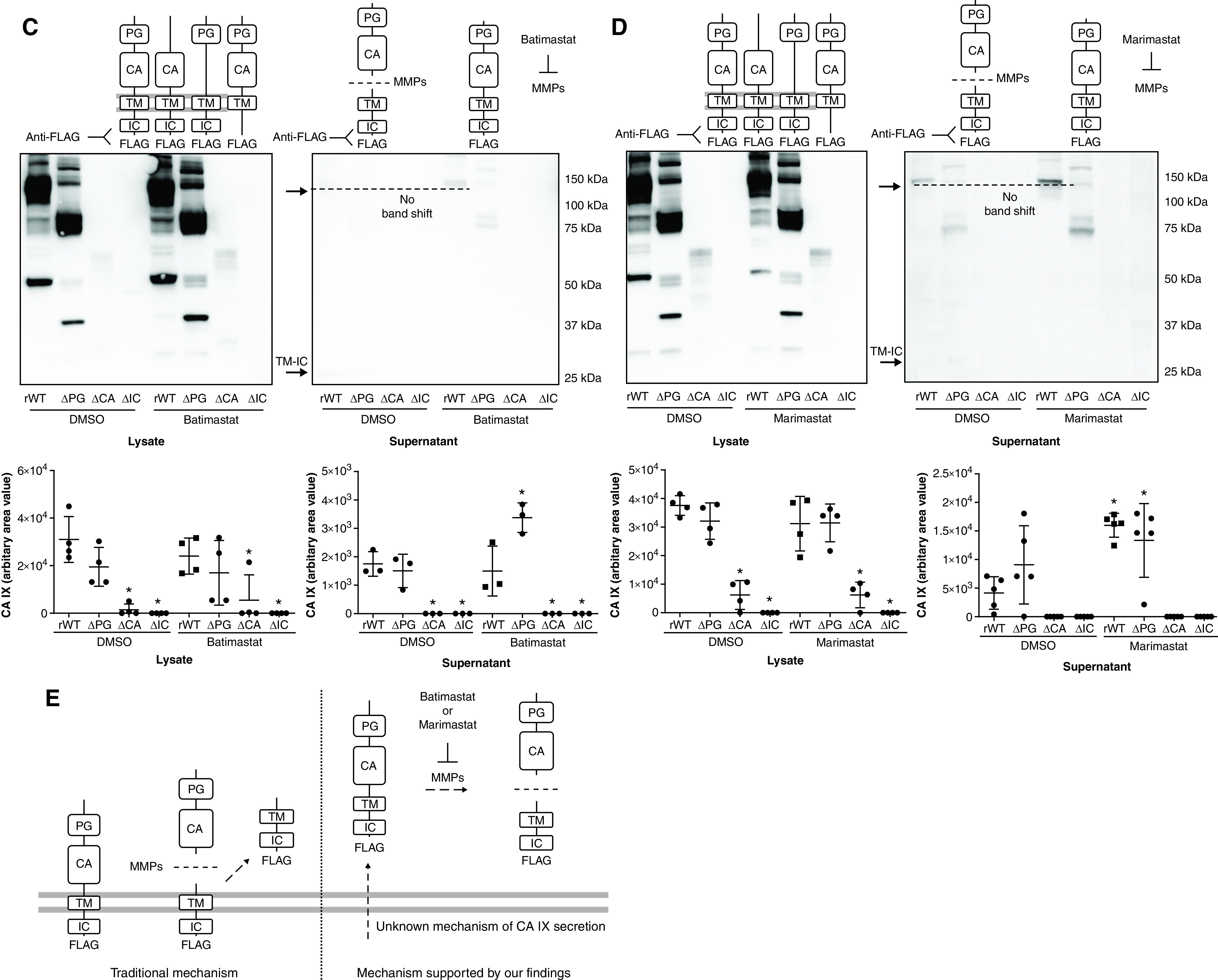
CA IX is released at full length and is cleaved extracellularly by MMPs (metalloproteinases) in PMVECs. (A–D) PMVEC CA IX mutant cell lines were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, media were changed to Hanks’ balanced salt solution (HBSS) and treated with DMSO (0.1%), batimastat (10μM), or marimastat (10μM). Twenty-four hours later, CA IX protein abundance was measured in cell lysates and supernatants by using Western blotting. (A and B) An M75 antibody was used to detect monomer and dimer band shifts in supernatants, whereas (C and D) a FLAG tag antibody was used to detect full-length CA IX and a new low-molecular-weight band. The CA IX band shift detected by using the M75 antibody and the appearance of a new low-molecular-weight band detected by using the FLAG tag antibody were inhibited by (A and C) batimastat or (B and D) marimastat treatment, indicating that the band shift and the appearance of a new low-molecular-weight band represent a cleaved PG–CA ectodomain and remnants of the TM–IC domain, respectively. (E) A potential mechanism of CA IX release and MMP-mediated ectodomain cleavage in PMVECs is schematically illustrated. Considering that MMP inhibitors only inhibit extracellular cleavage, as evidenced by no molecular weight shift in lysate CA IX and the presence of a TM–IC fragment only in supernatants but not in cell lysates, unprocessed release followed by extracellular ectodomain cleavage is most likely the mechanism of CA IX release in PMVECs. At least three separate experiments were performed. One-way ANOVA and Bonferroni post hoc tests used to compare different groups. *Significant difference (P < 0.05) from rWT DMSO. Readers may view the uncut gels for B and C in the data supplement.
